Chemical structures
- α-muricholic acid
- β-muricholic acid
- γ-muricholic acid (hyocholic acid)
- ω-muricholic acid
Muricholic acids are a group of bile acids found as some of the main forms in mice, which gives them their name, and at low concentrations in other species. [1]
The two principal forms, α- and β-muricholic acids, differ from the primary bile acids found in humans, cholic acid and chenodeoxycholic acid, by both having a hydroxyl group at the 6-position in the β-configuration. The orientation of the hydroxyl group at the 7-position defines whether they are α- or β-muricholic acid. Muricholic acids are detectable at low concentrations in human urine. [2]
The three major bile acids in germ-free mice are cholic acid, α-muricholic, and β-muricholic acids. [3] In conventional mice with a normal microbiome, ω-muricholic acid (with hydroxyls in the 6α- and 7β-positions), and various sulfated forms are also found. Conjugation with taurine (to give tauromuricholic acids which are the main forms), or with glycine (to give glycomuricholic acids) takes place in the liver before secretion.[ citation needed ]
The enzyme responsible for the 6-hydroxylation reactions forming muricholates in mice is the cytochrome P450 Cyp2c70. This produces α-muricholic acid from chenodeoxycholic acid, and β-muricholic acid from ursodeoxycholic acid. [4]
Tauromuricholic acids were shown to be potent antagonists of the bile acid receptor farnesoid X receptor (FXR). [5]

α-Ketoglutaric acid is an organic compound with the formula H2CC(O)(CH2)2CO2H). A white, nontoxic solid, it is a common dicarboxylic acid. Relevant to its biological roles, it exists in water as its conjugate base α-ketoglutarate. It is also classified as a 2-ketocarboxylic acid. β-Ketoglutaric acid is an isomer. "Ketoglutaric acid" and "ketoglutarate", when not qualified as α or β, almost always refers respectively to α-ketoglutaric acid or α-ketoglutarate.

Glucuronic acid is a uronic acid that was first isolated from urine. It is found in many gums such as gum arabic, xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals.

Cholic acid, also known as 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid is a primary bile acid that is insoluble in water, it is a white crystalline substance. Salts of cholic acid are called cholates. Cholic acid, along with chenodeoxycholic acid, is one of the two major bile acids produced by the liver, where it is synthesized from cholesterol. These two major bile acids are roughly equal in concentration in humans. Derivatives are made from cholyl-CoA, which exchanges its CoA with either glycine, or taurine, yielding glycocholic and taurocholic acid, respectively.

Chenodeoxycholic acid is a bile acid. Salts of this carboxylic acid are called chenodeoxycholates. Chenodeoxycholic acid is one of the main bile acids. It was first isolated from the bile of the domestic goose, which gives it the "cheno" portion of its name.

Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts.

The bile acid receptor (BAR), also known as farnesoid X receptor (FXR) or NR1H4, is a nuclear receptor that is encoded by the NR1H4 gene in humans.

The liver X receptor (LXR) is a member of the nuclear receptor family of transcription factors and is closely related to nuclear receptors such as the PPARs, FXR and RXR. Liver X receptors (LXRs) are important regulators of cholesterol, fatty acid, and glucose homeostasis. LXRs were earlier classified as orphan nuclear receptors, however, upon discovery of endogenous oxysterols as ligands they were subsequently deorphanized.

Cholesterol 7 alpha-hydroxylase also known as cholesterol 7-alpha-monooxygenase or cytochrome P450 7A1 (CYP7A1) is an enzyme that in humans is encoded by the CYP7A1 gene which has an important role in cholesterol metabolism. It is a cytochrome P450 enzyme, which belongs to the oxidoreductase class, and converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid synthesis.

UDP glucuronosyltransferase 2 family, polypeptide B4, also known as UGT2B4, is an enzyme that in humans is encoded by the UGT2B4 gene.

Organic solute transporter alpha, also known as OST-alpha, is a protein which in humans is encoded by the SLC51A gene.

Organic solute transporter beta, also known as OST-beta, is a protein which in humans is encoded by the OSTB gene.

CYP8B1 also known as sterol 12-alpha-hydroxylase is a protein which in humans is encoded by the CYP8B1 gene.

Hyodeoxycholic acid, also known as 3α,6α-Dihydroxy-5β-cholan-24-oic acid or HDCA, is a secondary bile acid, one of the metabolic byproducts of intestinal bacteria. It differs from deoxycholic acid in that the 6α-hydroxyl is in the 12 position in the former. The 6α-hydroxyl group makes HDCA a hydrophilic acid, a property it shares with hyocholic acid. HDCA is present in mammalian species in different proportions. It is the main acid constituent of hog bile, and for this reason it was used industrially as precursor for steroid synthesis before total synthesis became practical.

7α-Hydroxy-4-cholesten-3-one is an intermediate in the biochemical synthesis of bile acids from cholesterol. Its precursor, 7α-hydroxycholesterol, is produced from cholesterol by hepatic cholesterol 7α-hydroxylase (CYP7A1).
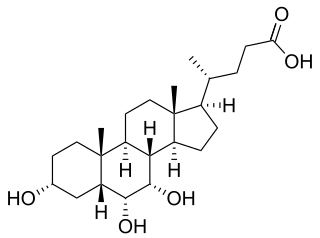
Hyocholic acid or 3α,6α,7α-trihydroxy-5β-cholan-24-oic acid is a bile acid found as one of the main forms in pig, and at low concentrations in other species including humans. Hyocholic acid differs from the primary bile acids found in humans by having a third hydroxyl group in the α-conformation at the 6-position, unlike cholic acid, which has a 12-hydroxyl, and chenodeoxycholic acid which has neither a 6- or 12-hydroxyl. It also differs from the muricholic acids found in rodents, as they are 6β-hydroxylated, and can have the 7-hydroxyl in either the α- or β- positions, forming α- or β-muricholic acids.
Fibroblast growth factor 15 is a protein in mouse encoded by the Fgf15 gene. It is a member of the fibroblast growth factor (FGF) family but, like FGF19, FGF21 and FGF23, has endocrine functions. FGF19 is the orthologous protein in humans. They are often referred together as FGF15/19.
Clostridium scindens is a Gram-positive, obligate anaerobic, pleiomorphic, spore-forming bacterium belonging to the genus Clostridium.C. scindens has been found in humans as a commensal colonizer of the colon. Clostridium scindens is capable of converting primary bile acids to secondary bile acids, as well as converting glucocorticoids to androgens. The presence of C. scindens in the human gut is associated resistance to Clostridioides difficile infection, due to the production of secondary bile acids which inhibit the growth of C. difficile.

Estradiol glucuronide, or estradiol 17β-D-glucuronide, is a conjugated metabolite of estradiol. It is formed from estradiol in the liver by UDP-glucuronyltransferase via attachment of glucuronic acid and is eventually excreted in the urine by the kidneys. It has much higher water solubility than does estradiol. Glucuronides are the most abundant estrogen conjugates.
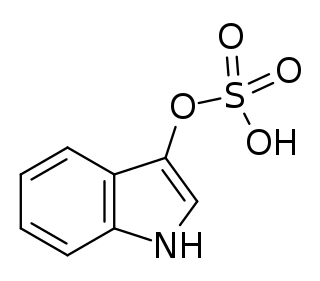
Indoxyl sulfate, also known as 3-indoxylsulfate and 3-indoxylsulfuric acid, is a metabolite of dietary L-tryptophan that acts as a cardiotoxin and uremic toxin. High concentrations of indoxyl sulfate in blood plasma are known to be associated with the development and progression of chronic kidney disease and vascular disease in humans. As a uremic toxin, it stimulates glomerular sclerosis and renal interstitial fibrosis.
Bile salt hydrolases (BSH) are microbial enzymes that deconjugate primary bile acids. They catalyze the first step of bile acid metabolism and maintain the bile acid pool for further modification by the microbiota. BSH enzymes play a role in a range of host and microbe functions including host physiology, immunity, and protection from pathogens.