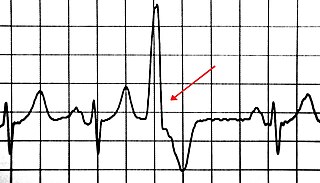
A premature ventricular contraction (PVC) is a relatively common event where the heartbeat is initiated by Purkinje fibers in the ventricles rather than by the sinoatrial node. PVCs may cause no symptoms or may be perceived as a "skipped beat" or felt as palpitations in the chest. Single beat PVCs do not usually pose a danger.

The endocardium is the innermost layer of tissue that lines the chambers of the heart. Its cells are embryologically and biologically similar to the endothelial cells that line blood vessels. The endocardium also provides protection to the valves and heart chambers.

Fibrosis, also known as fibrotic scarring, is a pathological wound healing in which connective tissue replaces normal parenchymal tissue to the extent that it goes unchecked, leading to considerable tissue remodelling and the formation of permanent scar tissue.

Intercalated discs are microscopic identifying features of cardiac muscle. Cardiac muscle consists of individual heart muscle cells (cardiomyocytes) connected by intercalated discs to work as a single functional syncytium. By contrast, skeletal muscle consists of multinucleated muscle fibers and exhibits no intercalated discs. Intercalated discs support synchronized contraction of cardiac tissue. They occur at the Z line of the sarcomere and can be visualized easily when observing a longitudinal section of the tissue.
In cardiology, ventricular remodeling refers to changes in the size, shape, structure, and function of the heart. This can happen as a result of exercise or after injury to the heart muscle. The injury is typically due to acute myocardial infarction, but may be from a number of causes that result in increased pressure or volume, causing pressure overload or volume overload on the heart. Chronic hypertension, congenital heart disease with intracardiac shunting, and valvular heart disease may also lead to remodeling. After the insult occurs, a series of histopathological and structural changes occur in the left ventricular myocardium that lead to progressive decline in left ventricular performance. Ultimately, ventricular remodeling may result in diminished contractile (systolic) function and reduced stroke volume.
Myocardial contractility represents the innate ability of the heart muscle (cardiac muscle or myocardium) to contract. The ability to produce changes in force during contraction result from incremental degrees of binding between different types of tissue, that is, between filaments of myosin (thick) and actin (thin) tissue. The degree of binding depends upon the concentration of calcium ions in the cell. Within an in vivo intact heart, the action/response of the sympathetic nervous system is driven by precisely timed releases of a catecholamine, which is a process that determines the concentration of calcium ions in the cytosol of cardiac muscle cells. The factors causing an increase in contractility work by causing an increase in intracellular calcium ions (Ca++) during contraction.

A myofibroblast is a cell phenotype that was first described as being in a state between a fibroblast and a smooth muscle cell.
Cardiac fibrosis commonly refers to the excess deposition of extracellular matrix in the cardiac muscle, but the term may also refer to an abnormal thickening of the heart valves due to inappropriate proliferation of cardiac fibroblasts. Fibrotic cardiac muscle is stiffer and less compliant and is seen in the progression to heart failure. The description below focuses on a specific mechanism of valvular pathology but there are other causes of valve pathology and fibrosis of the cardiac muscle.
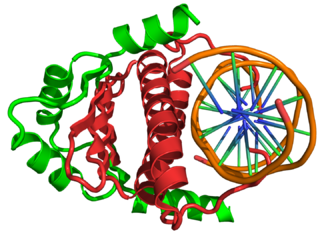
In the field of molecular biology, myocyte enhancer factor-2 (Mef2) proteins are a family of transcription factors which through control of gene expression are important regulators of cellular differentiation and consequently play a critical role in embryonic development. In adult organisms, Mef2 proteins mediate the stress response in some tissues. Mef2 proteins contain both MADS-box and Mef2 DNA-binding domains.

Diabetic cardiomyopathy is a disorder of the heart muscle in people with diabetes. It can lead to inability of the heart to circulate blood through the body effectively, a state known as heart failure, with accumulation of fluid in the lungs or legs. Most heart failure in people with diabetes results from coronary artery disease, and diabetic cardiomyopathy is only said to exist if there is no coronary artery disease to explain the heart muscle disorder.

A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may travel into the shoulder, arm, back, neck or jaw. Often it occurs in the center or left side of the chest and lasts for more than a few minutes. The discomfort may occasionally feel like heartburn. Other symptoms may include shortness of breath, nausea, feeling faint, a cold sweat or feeling tired. About 30% of people have atypical symptoms. Women more often present without chest pain and instead have neck pain, arm pain or feel tired. Among those over 75 years old, about 5% have had an MI with little or no history of symptoms. An MI may cause heart failure, an irregular heartbeat, cardiogenic shock or cardiac arrest.

Myocardial scarring is the accumulation of fibrosis tissue resulting after some form of trauma to the cardiac tissue. Fibrosis is the formation of excess tissue in replacement of necrotic or extensively damaged tissue. Fibrosis in the heart is often hard to detect because fibromas are often formed. Fibromas are scar tissue or small tumors, formed in one cell line. Because they are so small they can be hard to detect by methods such as magnetic resonance imaging. A cell line is a path of fibrosis that follow only a line of cells.
Myocardial infarction complications may occur immediately following a heart attack, or may need time to develop. After an infarction, an obvious complication is a second infarction, which may occur in the domain of another atherosclerotic coronary artery, or in the same zone if there are any live cells left in the infarct.
A diagnosis of myocardial infarction is created by integrating the history of the presenting illness and physical examination with electrocardiogram findings and cardiac markers. A coronary angiogram allows visualization of narrowings or obstructions on the heart vessels, and therapeutic measures can follow immediately. At autopsy, a pathologist can diagnose a myocardial infarction based on anatomopathological findings.
Cenderitide is a natriuretic peptide developed by the Mayo Clinic as a potential treatment for heart failure. Cenderitide is created by the fusion of the 15 amino acid C-terminus of dendroaspis natriuretic peptide (DNP) with the full C-type natriuretic peptide (CNP) structure both peptide which are endogenous to humans. This peptide chimera is a dual activator of the natriuretic peptide receptors NPR-A and NPR-B and therefore exhibits the natriuretic and diuretic properties of DNP, as well as the antiproliferative and antifibrotic properties of CNP.
Heart nanotechnology is the "Engineering of functional systems at the molecular scale".
Human HGF plasmid DNA therapy of cardiomyocytes is being examined as a potential treatment for coronary artery disease, as well as treatment for the damage that occurs to the heart after MI. After MI, the myocardium suffers from reperfusion injury which leads to death of cardiomyocytes and detrimental remodelling of the heart, consequently reducing proper cardiac function. Transfection of cardiac myocytes with human HGF reduces ischemic reperfusion injury after MI. The benefits of HGF therapy include preventing improper remodelling of the heart and ameliorating heart dysfunction post-MI.

Oxymatrine is one of many quinolizidine alkaloid compounds extracted from the root of Sophora flavescens, a Chinese herb. It is very similar in structure to matrine, which has one less oxygen atom. Oxymatrine has a variety of effects in vitro and in animal models, including protection against apoptosis, tumor and fibrotic tissue development, and inflammation. Furthermore, oxymatrine has been shown to decrease cardiac ischemia, myocardial injury, arrhythmias, and improve heart failure by increasing cardiac function.
Tissue remodeling is the reorganization or renovation of existing tissues. Tissue remodeling can be either physiological or pathological. The process can either change the characteristics of a tissue such as in blood vessel remodeling, or result in the dynamic equilibrium of a tissue such as in bone remodeling. Macrophages repair wounds and remodel tissue by producing extracellular matrix and proteases to modify that specific matrix.
Milica Radisic is a Serbian Canadian tissue engineer, academic and researcher. She is a Professor at the University of Toronto’s Institute of Biomaterials and Biomedical Engineering, and the Department of Chemical Engineering and Applied Chemistry. She co-founded TARA Biosystems and is a senior scientist at the Toronto General Hospital Research Institute.









