Related Research Articles
In cellular biology, P-bodies, or processing bodies, are distinct foci formed by phase separation within the cytoplasm of a eukaryotic cell consisting of many enzymes involved in mRNA turnover. P-bodies are highly conserved structures and have been observed in somatic cells originating from vertebrates and invertebrates, plants and yeast. To date, P-bodies have been demonstrated to play fundamental roles in general mRNA decay, nonsense-mediated mRNA decay, adenylate-uridylate-rich element mediated mRNA decay, and microRNA (miRNA) induced mRNA silencing. Not all mRNAs which enter P-bodies are degraded, as it has been demonstrated that some mRNAs can exit P-bodies and re-initiate translation. Purification and sequencing of the mRNA from purified processing bodies showed that these mRNAs are largely translationally repressed upstream of translation initiation and are protected from 5' mRNA decay.

In the cellular biology, stress granules are biomolecular condensates in the cytosol composed of proteins and RNA that assemble into 0.1–2 μm membraneless organelles when the cell is under stress. The mRNA molecules found in stress granules are stalled translation pre-initiation complexes associated with 40S ribosomal subunits, translation initiation factors, poly(A)+ mRNA and RNA-binding proteins (RBPs). While they are membraneless organelles, stress granules have been proposed to be associated with the endoplasmatic reticulum. There are also nuclear stress granules. This article is about the cytosolic variety.
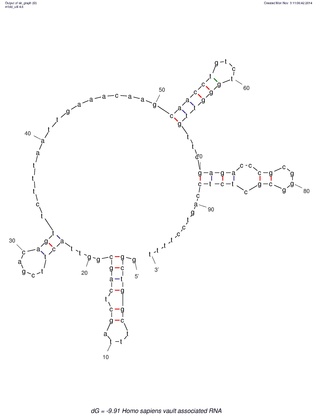
Many eukaryotic cells contain large ribonucleoprotein particles in the cytoplasm known as vaults. The vault complex comprises the major vault protein (MVP), two minor vault proteins, and a variety of small untranslated RNA molecules known as vault RNAs only found in higher eukaryotes. These molecules are transcribed by RNA polymerase III.
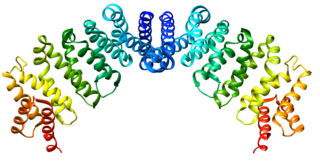
Exportin 1 (XPO1), also known as chromosomal region maintenance 1 (CRM1), is a eukaryotic protein that mediates the nuclear export of various proteins and RNAs.

Poly [ADP-ribose] polymerase 4 is an enzyme that in humans is encoded by the PARP4 gene.

The vault or vault cytoplasmic ribonucleoprotein is a eukaryotic organelle whose function is not yet fully understood. Discovered and isolated by Nancy Kedersha and Leonard Rome in 1986, vaults are cytoplasmic organelles which, when negative-stained and viewed under an electron microscope, resemble the arches of a cathedral's vaulted ceiling, with 39-fold symmetry. They are present in many types of eukaryotic cells, and appear to be highly conserved among eukaryotes.

Jennifer Lippincott-Schwartz is a Senior Group Leader at Howard Hughes Medical Institute's Janelia Research Campus and a founding member of the Neuronal Cell Biology Program at Janelia. Previously, she was the Chief of the Section on Organelle Biology in the Cell Biology and Metabolism Program, in the Division of Intramural Research in the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health from 1993 to 2016. Lippincott-Schwartz received her PhD from Johns Hopkins University, and performed post-doctoral training with Richard Klausner at the NICHD, NIH in Bethesda, Maryland.
Leonard H. Rome is a cell biologist and biochemist who has been a faculty member of the David Geffen School of Medicine at UCLA, since he joined the Department of Biological Chemistry there, in 1979. He became a full professor in 1988 and has also served as the Senior Associate Dean for Research in the Geffen School of Medicine from 1997 to 2012. He is the Associate Director of the California NanoSystems Institute (CNSI) since 2004, and was Interim Director from 2007-2009. In addition, he served from 2001 to 2005 as University of California, Los Angeles (UCLA) Associate Vice Chancellor for Research for the Life and Health Sciences.
Conly Leroy Rieder is a cancer researcher in the field of mitotic cellular division, the study of cell division processes and cancer pathology. He conducted research at the Wadsworth Center, part of the New York State Department of Health, in Albany, New York. His published literature has discussed chromosome motility, spindle assembly, and mitotic checkpoints.

Anna Sergeevna Akhmanova is a Russian-born professor of Cell Biology at Utrecht University in the Netherlands. She is best known for her research regarding microtubules and the proteins, called TIPs, that stabilize one specific end of the tubules. Among the awards she has won, she was one of the recipients of the 2018 Spinoza Prize, the highest honor for Dutch scientists.

Amy S. Gladfelter is an American quantitative cell biologist who is interested in understanding fundamental mechanisms of cell organization. She was a Professor of Biology and the Associate Chair for Diversity Initiatives at the University of North Carolina at Chapel Hill, before moving to Department of Cell Biology at Duke University. She investigates cell cycle control and the septin cytoskeleton. She is also affiliated with the Lineberger Comprehensive Cancer Center and is a fellow of the Marine Biological Laboratory in Woods Hole, MA.
Ruth Lehmann is a developmental and cell biologist. She is the Director of the Whitehead Institute for Biomedical Research. She previously was affiliated with the New York University School of Medicine, where she was the Director of the Skirball Institute of Biomolecular Medicine, the Laura and Isaac Perlmutter Professor of Cell Biology, and the Chair of the Department of Cell Biology. Her research focuses on germ cells and embryogenesis.

Nancy Hogg FMedSci is an immunologist who has made major contributions in the field of adhesion molecules, focusing on the integrins expressed by leukocytes. Hogg was elected to the Academy of Medical Sciences in 2002 and currently holds an emeritus position at the Francis Crick Institute, London.
Samara Reck-Peterson is an American cell biologist and biophysicist. She is a Professor of Cellular and Molecular Medicine and Cell and Developmental Biology at the University of California, San Diego and an Investigator of the Howard Hughes Medical Institute. She is known for her contributions to our understanding of how dynein, an exceptionally large motor protein that moves many intracellular cargos, works and is regulated. She developed one of the first systems to produce recombinant dynein and discovered that, unlike other cytoskeletal motors, dynein can take a wide variety of step sizes, forward and back and even sideways. She lives in San Diego, California.

Rebecca W. Heald is an American professor of cell and developmental biology. She is currently a Professor in the Department of Molecular & Cell Biology at the University of California, Berkeley. In May 2019, she was elected to the National Academy of Sciences. She has published over 120 research articles in peer reviewed journals.

Jean Gruenberg is a Swiss biologist, and a professor at the University of Geneva. His research in the fields of cell biology and biochemistry has significantly contributed to a better understanding of the molecular mechanisms involved in the intracellular traffic within eukaryotic cells, more especially in the endolysosomal pathway.
Lucas Andrew Staehelin was a retired Swiss-American cell biologist. He was professor emeritus at the University of Colorado Boulder.
Jennifer Waters is an American scientist who is a Lecturer on Cell Biology, the Director of the Core for Imaging Technology & Education(CITE; formally the NIC) and the Director of the Cell Biology Microscopy Facility at Harvard Medical School. She is an imaging expert and educator whose efforts to educate life scientists about microscopy and to systemize the education of microscopists in microscopy facilities serve as a blueprint for similar efforts worldwide.

Gia Voeltz is an American cell biologist. She is a professor of Molecular, Cellular and Developmental Biology at the University of Colorado Boulder and a Howard Hughes Medical Institute Investigator. She is known for her research identifying the factors and unraveling the mechanisms that determine the structure and dynamics of the largest organelle in the cell: the endoplasmic reticulum. Her lab has produced paradigm shifting studies on organelle membrane contact sites that have revealed that most cytoplasmic organelles are not isolated entities but are instead physically tethered to an interconnected ER membrane network.
Nancy Lane Perham is a Canadian cell biologist and artist ,and is a full professor at the University of Cambridge, specialising in cell-to-cell interactions.
References
- ↑ "Richard Kedersha Obituary (2008) FloridaToday". Legacy.com. Retrieved 2021-09-28.
- ↑ "Rutherford is looking for distinguished graduates to add to list", South Bergenite, January 29, 2003. Accessed September 29, 2021, via Newspapers.com. "cell biologist Nancy Kedersha, '69, whose examination of the components of a cell led to the identification of a new organelle named 'vault,' 1997."
- 1 2 "Welcome to the Vault Website: Maintained by the Rome Laboratory at UCLA: OPEN_CMS". vaults.arc.ucla.edu. Retrieved 2021-09-29.
- ↑ Berg, R. A.; Kedersha, N. L.; Guzman, N. A. (1979-04-25). "Purification and partial characterization of the two nonidentical subunits of prolyl hydroxylase". The Journal of Biological Chemistry. 254 (8): 3111–3118. doi: 10.1016/S0021-9258(17)30189-8 . ISSN 0021-9258. PMID 218963.
- ↑ Kedersha, N. L.; Berg, R. A. (July 1981). "An improved method for the purification of vertebrate prolyl hydroxylase by affinity chromatography". Collagen and Related Research. 1 (4): 345–353. doi:10.1016/s0174-173x(81)80011-8. ISSN 0174-173X. PMID 6286234.
- ↑ Kedersha, N. L.; Tkacz, J. S.; Berg, R. A. (1985-10-08). "Biosynthesis of prolyl hydroxylase: evidence for two separate dolichol-media pathways of glycosylation". Biochemistry. 24 (21): 5960–5967. doi:10.1021/bi00342a041. ISSN 0006-2960. PMID 3002430.
- 1 2 3 "Lennart Nilsson Award 2011: The Secret Life of Cells – in Colour". Wiley Analytical Science. doi:10.1002/imaging.2768 (inactive 1 November 2024). Retrieved 2021-09-29.
{{cite web}}: CS1 maint: DOI inactive as of November 2024 (link) - ↑ Kedersha, N. L.; Rome, L. H. (1986-09-01). "Isolation and characterization of a novel ribonucleoprotein particle: large structures contain a single species of small RNA". The Journal of Cell Biology. 103 (3): 699–709. doi:10.1083/jcb.103.3.699. ISSN 0021-9525. PMC 2114306 . PMID 2943744.
- ↑ Kedersha, N. L.; Miquel, M. C.; Bittner, D.; Rome, L. H. (1990-04-01). "Vaults. II. Ribonucleoprotein structures are highly conserved among higher and lower eukaryotes". The Journal of Cell Biology. 110 (4): 895–901. doi:10.1083/jcb.110.4.895. ISSN 0021-9525. PMC 2116106 . PMID 1691193.
- ↑ Kolev, Nikolay G.; Rajan, K. Shanmugha; Tycowski, Kazimierz T.; Toh, Justin Y.; Shi, Huafang; Lei, Yuling; Michaeli, Shulamit; Tschudi, Christian (2019-10-25). "The vault RNA of Trypanosoma brucei plays a role in the production of trans-spliced mRNA". The Journal of Biological Chemistry. 294 (43): 15559–15574. doi: 10.1074/jbc.RA119.008580 . ISSN 0021-9258. PMC 6816085 . PMID 31439669.
- ↑ Kedersha, N. L.; Gupta, M.; Li, W.; Miller, I.; Anderson, P. (1999-12-27). "RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules". The Journal of Cell Biology. 147 (7): 1431–1442. doi:10.1083/jcb.147.7.1431. ISSN 0021-9525. PMC 2174242 . PMID 10613902.
- ↑ Kedersha, Nancy; Stoecklin, Georg; Ayodele, Maranatha; Yacono, Patrick; Lykke-Andersen, Jens; Fritzler, Marvin J.; Scheuner, Donalyn; Kaufman, Randal J.; Golan, David E.; Anderson, Paul (2005-06-20). "Stress granules and processing bodies are dynamically linked sites of mRNP remodeling". The Journal of Cell Biology. 169 (6): 871–884. doi:10.1083/jcb.200502088. ISSN 0021-9525. PMC 2171635 . PMID 15967811.
- ↑ Kedersha, Nancy; Panas, Marc D.; Achorn, Christopher A.; Lyons, Shawn; Tisdale, Sarah; Hickman, Tyler; Thomas, Marshall; Lieberman, Judy; McInerney, Gerald M.; Ivanov, Pavel; Anderson, Paul (2016-03-28). "G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits". The Journal of Cell Biology. 212 (7): 845–860. doi:10.1083/jcb.201508028. ISSN 1540-8140. PMC 4810302 . PMID 27022092.
- ↑ "Translation Mechanisms and Control". cshperspectives.cshlp.org. Retrieved 2021-09-28.
- ↑ "Nancy Kedersha". Nikon’s Small World. Retrieved 2021-09-28.
- ↑ "The secret life of cells – in colour". www.bionity.com. Retrieved 2021-09-28.
- ↑ "Fotografen Lennart Nilsson och Lennart Nilsson Award". Lennartnilssonaward.se (in Swedish). Retrieved 2021-09-29.