
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range (nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:

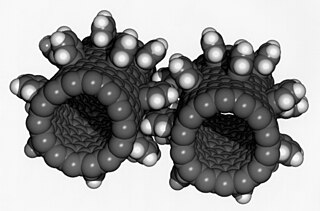
A fullerene is an allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may have hollow sphere- and ellipsoid-like forms, tubes, or other shapes.
Nanotechnology is the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). At this scale, commonly known as the nanoscale, surface area and quantum mechanical effects become important in describing properties of matter. This definition of nanotechnology includes all types of research and technologies that deal with these special properties. It is common to see the plural form "nanotechnologies" as well as "nanoscale technologies" to refer to research and applications whose common trait is scale. An earlier understanding of nanotechnology referred to the particular technological goal of precisely manipulating atoms and molecules for fabricating macroscale products, now referred to as molecular nanotechnology.

β-Carbon nitride (beta-carbon nitride), β-C3N4, is a superhard material predicted to be harder than diamond.

Aluminium nitride (AlN) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies.
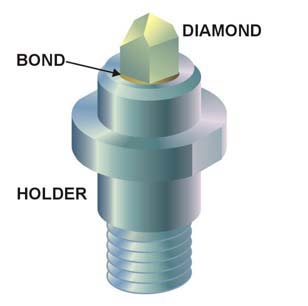
A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.
In chemistry, a nitride is a chemical compound of nitrogen. Nitrides can be inorganic or organic, ionic or covalent. The nitride anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitrides have a found applications, such as wear-resistant coatings (e.g., titanium nitride, TiN), hard ceramic materials (e.g., silicon nitride, Si3N4), and semiconductors (e.g., gallium nitride, GaN). The development of GaN-based light emitting diodes was recognized by the 2014 Nobel Prize in Physics. Metal nitrido complexes are also common.
A non-carbon nanotube is a cylindrical molecule often composed of metal oxides, or group III-Nitrides and morphologically similar to a carbon nanotube. Non-carbon nanotubes have been observed to occur naturally in some mineral deposits.

Nanocomposite is a multiphase solid material where one of the phases has one, two or three dimensions of less than 100 nanometers (nm) or structures having nano-scale repeat distances between the different phases that make up the material.
Alex K. Zettl is an American experimental physicist, educator, and inventor.

National Institute for Materials Science is an Independent Administrative Institution and one of the largest scientific research centers in Japan.

The optical properties of carbon nanotubes are highly relevant for materials science. The way those materials interact with electromagnetic radiation is unique in many respects, as evidenced by their peculiar absorption, photoluminescence (fluorescence), and Raman spectra.
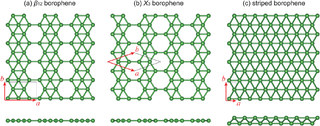
Borophene is a crystalline atomic monolayer of boron, i.e., it is a two-dimensional allotrope of boron and also known as boron sheet. First predicted by theory in the mid-1990s, different borophene structures were experimentally confirmed in 2015.

Boron nitride nanotubes (BNNTs) are a polymorph of boron nitride. They were predicted in 1994 and experimentally discovered in 1995. Structurally they are similar to carbon nanotubes, which are cylinders with sub-micrometer diameters and micrometer lengths, except that carbon atoms are alternately substituted by nitrogen and boron atoms. However, the properties of BN nanotubes are very different: whereas carbon nanotubes can be metallic or semiconducting depending on the rolling direction and radius, a BN nanotube is an electrical insulator with a bandgap of ~5.5 eV, basically independent of tube chirality and morphology. In addition, a layered BN structure is much more thermally and chemically stable than a graphitic carbon structure. BNNTs have unique physical and chemical properties, when compared to Carbon Nanotubes (CNTs) providing a very wide range of commercial and scientific applications. Although BNNTs and CNTs share similar tensile strength properties of circa 100 times stronger than steel and 50 times stronger than industrial-grade carbon fibre, BNNTs can withstand high temperatures of up to 900 °C. as opposed to CNTs which remain stable up to temperatures of 400 °C, and are also capable of absorbing radiation. BNNTS are packed with physicochemical features including high hydrophobicity and considerable hydrogen storage capacity and they are being investigated for possible medical and biomedical applications, including gene delivery, drug delivery, neutron capture therapy, and more generally as biomaterials BNNTs are also superior to CNTs in the way they bond to polymers giving rise to many new applications and composite materials.
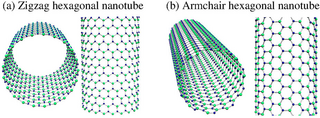
Gallium nitride nanotubes (GaNNTs) are nanotubes of gallium nitride. They can be grown by chemical vapour deposition.
Graphene-Boron Nitride nanohybrid materials are a class of compounds created from graphene and boron nitride nanosheets. Graphene and boron nitride both contain intrinsic thermally conductive and electrically insulative properties. The combination of these two compounds may be useful to advance the development and understanding of electronics.

Boron nitride nanosheet is a crystalline form of the hexagonal boron nitride (h-BN), which has a thickness of one atom. Similar in geometry as well as physical and thermal properties to its carbon analog graphene, but has very different chemical and electronic properties – contrary to the black and highly conducting graphene, BN nanosheets are electrical insulators with a band gap of ~5.9 eV, and therefore appear white in color.

Sphere packing in a cylinder is a three-dimensional packing problem with the objective of packing a given number of identical spheres inside a cylinder of specified diameter and length. For cylinders with diameters on the same order of magnitude as the spheres, such packings result in what are called columnar structures.
Yoke Khin Yap is an American physicist, materials scientist and academic. He is most known for his nanoscale and quantum-scale materials research, and serves as a professor of Physics at Michigan Technological University (MTU).















