Chemical structures
- Naphthomycin A
- Naphthomycin B
- Naphthomycin C
- Naphthomycin D
- Naphthomycin E
- Naphthomycin F
- Naphthomycin G
Naphthomycins are a group of closely related antimicrobial chemical compounds isolated from Streptomyces . They are considered a subclass of ansamycins.
Members include:

Nonactin is a member of a family of naturally occurring cyclic ionophores known as the macrotetrolide antibiotics. The other members of this homologous family are monactin, dinactin, trinactin and tetranactin which are all neutral ionophoric substances and higher homologs of nonactin. Collectively, this class is known as the nactins. Nonactin is soluble in methanol, dichloromethane, ethyl acetate and DMSO, but insoluble in water.

Streptomyces is the largest genus of Actinomycetota and the type genus of the family Streptomycetaceae. Over 500 species of Streptomyces bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin.

Viomycin is a member of the tuberactinomycin family, a group of nonribosomal peptide antibiotics exhibiting anti-tuberculosis activity. The tuberactinomycin family is an essential component in the drug cocktail currently used to fight infections of Mycobacterium tuberculosis. Viomycin was the first member of the tuberactinomycins to be isolated and identified, and was used to treat TB until it was replaced by the less toxic, but structurally related compound, capreomycin. The tuberactinomycins target bacterial ribosomes, binding RNA and disrupting bacterial protein synthesis and certain forms of RNA splicing. Viomycin is produced by the actinomycete Streptomyces puniceus.

Boromycin is a bacteriocidal polyether-macrolide antibiotic. It was initially isolated from the Streptomyces antibioticus, and is notable for being the first natural product found to contain the element boron. It is effective against most Gram-positive bacteria, but is ineffective against Gram-negative bacteria. Boromycin kills bacteria by negatively affecting the cytoplasmic membrane, resulting in the loss of potassium ions from the cell.

Ansamycins is a family of bacterial secondary metabolites that show antimicrobial activity against many Gram-positive and some Gram-negative bacteria, and includes various compounds, including streptovaricins and rifamycins. In addition, these compounds demonstrate antiviral activity towards bacteriophages and poxviruses.
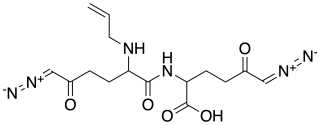
Alazopeptin is an antibiotic, with moderate anti-trypanosomal and antitumor activity. It was originally isolated from Streptacidiphilus griseoplanus, sourced from soil near Williamsburg, Iowa. It is also isolated from Kitasatospora azatica. It is still largely produced via fermentation broths of that organism. Structurally, alazopeptin is a tripeptide and contains 2 molecules of 6-diazo-5-oxo-L-norleucine and one molecule of L-alanine. In 2021 the biosynthetic pathway of alazopeptin was elucidated.

Callystatin A is a polyketide natural product from the leptomycin family of secondary metabolites. It was first isolated in 1997 from the marine sponge Callyspongia truncata which was collected from the Goto Islands in the Nagasaki Prefecture of Japan by the Kobayashi group. Since then its absolute configuration has been elucidated and callystatin A was discovered to have anti-fungal and anti-tumor activities with extreme potency against the human epidermoid carcinoma KB cells (IG50 = 10 pg/ml) and the mouse lymphocytic leukemia Ll210 cells (IG50 = 20 pg/ml).

Naphthomycin A is a type of naphthomycin. It was isolated as a yellow pigment from Streptomyces collinus and it shows antibacterial, antifungal, and antitumor activities. Naphthomycins have the longest ansa aliphatic carbon chain of the ansamycin family. Biosynthetic origins of the carbon skeleton from PKS1 were investigated by feeding 13C-labeled precursors and subsequent 13C-NMR product analysis. Naphthomycin gene clusters have been cloned and sequenced to confirm involvement in biosynthesis via deletion of a 7.2kb region. Thirty-two genes were identified in the 106kb cluster.

Germicidins are a groups of natural products arising from Streptomyces species that acts as autoregulatory inhibitor of spore germination. In Streptomyces viriochromogenes, low concentrations inhibit germination of its own arthrospores, and higher concentrations inhibit porcine Na+/K+ -activated ATPase. Inhibitory effects on germination are also observed when germicidin from Streptomyces is applied to Lepidium sativum. Germicidins and other natural products present potential use as pharmaceuticals, and in this case, those with possible antibiotic or antifungal activity.
Venturicidins are a group of antifungal compounds. The first member of this class was isolated from Streptomyces bacteria in 1961. Additional members of this class were subsequently isolated and characterized. An antifungal substance "aabomycin A" was isolated from Streptomyces in 1969. However, in 1990 it was reported that aabomycin A is actually a 3:1 mixture of two related components, which were then named aabomycin A1 and aabomycin A2. The structures of these were shown to be identical with those of the previously characterized compounds venturicidin A and venturicidin B, respectively. A new analog, venturicidin C, was recently reported from a Streptomyces isolated from thermal vents associated with the Ruth Mullins coal fire in Kentucky.
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.
Streptomyces aurantiacus is a bacterium species from the genus Streptomyces which produces aurantin, pamamycin-621, aurantimycin A, aurantimycin B, aurantimycin C, aurantimycin D, dihydronancimycin and ancimycin.
Streptomyces diastatochromogenes is a bacterium species from the genus of Streptomyces. Streptomyces diastatochromogenes produces polyketomycin, concanamycin A, concanamycin B, concanamycin C, momofulvenone A, azdimycin, toyocamycin and oligomycins.
Streptomyces griseoaurantiacus is a thermotolerant bacterium species from the genus of Streptomyces which was isolated from marine sediment. Streptomyces griseoaurantiacus produces the antibiotics manumycin, diperamycin and chinikomycin, and griseolic acid.
Streptomyces griseoflavus is a bacterium species from the genus of Streptomyces which has been isolated from garden soil. Streptomyces griseoflavus produces bicozamycin, colabomycins A, colabomycins C, germacradienol and hormaomycin.
Streptomyces microflavus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces microflavus produces nemadectin, fattiviracin A1, milbemycin and deoxyuridines. Streptomyces microflavus also produces the ionophore valinomycin. Streptomyces microflavus is also known to cause potato common scab disease in Korea.
Streptomyces sanglieri is a bacterium species from the genus of Streptomyces which has been isolated from soil from a hay meadow. Streptomyces sanglieri produces the antibiotic lactonamycin Z.
Streptomyces violaceusniger is a bacterium species from the genus of Streptomyces. Streptomyces violaceusniger has antifungal activity. Streptomyces violaceusniger produces isoafricanol and spirofungin.
Streptomyces lasiicapitis is a bacterium species from the genus of Streptomyces which has been isolated from the head of the ant Lasius fuliginosus. Streptomyces lasiicapitis produces the antibiotic kanchanamycin.

Tetracenomycin C is an antitumor anthracycline-like antibiotic produced by Streptomyces glaucescens GLA.0. The pale-yellow antibiotic is active against some gram-positive bacteria, especially against streptomycetes. Gram-negative bacteria and fungi are not inhibited. In considering the differences of biological activity and the functional groups of the molecule, tetracenomycin C is not a member of the tetracycline or anthracyclinone group of antibiotics. Tetracenomycin C is notable for its broad activity against actinomycetes. As in other anthracycline antibiotics, the framework is synthesized by a polyketide synthase and subsequently modified by other enzymes.