Related Research Articles

Gene therapy is a medical technology that aims to produce a therapeutic effect through the manipulation of gene expression or through altering the biological properties of living cells.

A genetic chimerism or chimera is a single organism composed of cells with more than one distinct genotype. Animal chimeras can be produced by the merger of two embryos. In plants and some animal chimeras, mosaicism involves distinct types of tissue that originated from the same zygote but differ due to mutation during ordinary cell division.

Transplant rejection occurs when transplanted tissue is rejected by the recipient's immune system, which destroys the transplanted tissue. Transplant rejection can be lessened by determining the molecular similitude between donor and recipient and by use of immunosuppressant drugs after transplant.

A germline mutation, or germinal mutation, is any detectable variation within germ cells. Mutations in these cells are the only mutations that can be passed on to offspring, when either a mutated sperm or oocyte come together to form a zygote. After this fertilization event occurs, germ cells divide rapidly to produce all of the cells in the body, causing this mutation to be present in every somatic and germline cell in the offspring; this is also known as a constitutional mutation. Germline mutation is distinct from somatic mutation.
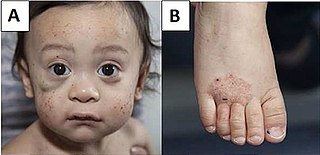
Wiskott–Aldrich syndrome (WAS) is a rare X-linked recessive disease characterized by eczema, thrombocytopenia, immune deficiency, and bloody diarrhea. It is also sometimes called the eczema-thrombocytopenia-immunodeficiency syndrome in keeping with Aldrich's original description in 1954. The WAS-related disorders of X-linked thrombocytopenia (XLT) and X-linked congenital neutropenia (XLN) may present with similar but less severe symptoms and are caused by mutations of the same gene.
Cord blood is blood that remains in the placenta and in the attached umbilical cord after childbirth. Cord blood is collected because it contains stem cells, which can be used to treat hematopoietic and genetic disorders such as cancer.
Gene trapping is a high-throughput approach that is used to introduce insertional mutations across an organism's genome.

Tumor protein p63, typically referred to as p63, also known as transformation-related protein 63, is a protein that in humans is encoded by the TP63 gene.

Fms-related tyrosine kinase 3 ligand (FLT3LG) is a protein which in humans is encoded by the FLT3LG gene.

The James H. Clark Center at Stanford University, California, United States, is a building, completed in 2003, that houses interdisciplinary research in the biological sciences.

Huda Yahya Zoghbi is a Lebanese-born American geneticist, and a professor at the Departments of Molecular and Human Genetics, Neuroscience and Neurology at the Baylor College of Medicine. She is the director of the Jan and Dan Duncan Neurological Research Institute. She was the editor of the Annual Review of Neuroscience from 2018-2024.

Genetic engineering techniques allow the modification of animal and plant genomes. Techniques have been devised to insert, delete, and modify DNA at multiple levels, ranging from a specific base pair in a specific gene to entire genes. There are a number of steps that are followed before a genetically modified organism (GMO) is created. Genetic engineers must first choose what gene they wish to insert, modify, or delete. The gene must then be isolated and incorporated, along with other genetic elements, into a suitable vector. This vector is then used to insert the gene into the host genome, creating a transgenic or edited organism.

Tetratricopeptide repeat domain 7A (TTC7A) is a protein that in humans is encoded by the TTC7A gene.

Diana W. Bianchi is the director of the U.S. National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development, a post often called “the nation’s pediatrician.” She is a medical geneticist and neonatologist noted for her research on fetal cell microchimerism and prenatal testing. Bianchi had previously been the Natalie V. Zucker Professor of Pediatrics, Obstetrics, and Gynecology at Tufts University School of Medicine and founder and executive director of the Mother Infant Research Institute at Tufts Medical Center. She also has served as Vice Chair for Research in the Department of Pediatrics at the Floating Hospital for Children at Tufts Medical Center.

Margaret ("Peggy") A. Goodell is an American scientist working in the field of stem cell research. Dr. Goodell is Chair of the Department of Molecular and Cellular Biology at Baylor College of Medicine, Director of the Stem Cell and Regenerative Medicine (STaR) Center, and a member of the National Academy of Medicine. She is best known for her contributions to understanding how blood stem cells are regulated.
Gene therapy for blood diseases is a novel field of research investigating ways in which components of blood can be genetically modified to treat hematologic diseases.

Sergiu P. Pașca is a Romanian-American scientist and physician at Stanford University in California. He is renowned for his groundbreaking work creating and developing stem cell-based models of the human brain to gain insights into neuropsychiatric disease. His lab was the first to develop and name assembloids: multi-unit self-organizing structures created in 3D cultures that allow for the study of human neural circuit and systems functions in vitro. Pașca’s lab generated and published human cortico-striatal and cortico-motor assembloids in 2020. Combining regionalized neural organoids pioneered in the lab and studies with human forebrain assembloids and transplantation, in 2024, Pașca developed a therapeutic for a severe genetic disorder called Timothy Syndrome, which was published on the cover of Nature.

The He Jiankui genome editing incident is a scientific and bioethical controversy concerning the use of genome editing following its first use on humans by Chinese scientist He Jiankui, who edited the genomes of human embryos in 2018. He became widely known on 26 November 2018 after he announced that he had created the first human genetically edited babies. He was listed in Time magazine's 100 most influential people of 2019. The affair led to ethical and legal controversies, resulting in the indictment of He and two of his collaborators, Zhang Renli and Qin Jinzhou. He eventually received widespread international condemnation.

Crystal L. Mackall is an American physician and immunologist. She is currently the Ernest and Amelia Gallo Family Professor of Pediatrics and Medicine at Stanford University. She is the founding director of the Stanford Center for Cancer Cell Therapy.
Maria Grazia Roncarolo is an Italian pediatrician who is currently George D. Smith Professor in Stem Cell and Regenerative Medicine and Professor of Medicine at Stanford University. She is also the Director of the Stanford Institute of Stem Cell Biology and Regenerative Medicine along with Irving Weissman and Michael Longaker and the Director for Center for Definitive and Curative Medicine at Stanford.
References
- 1 2 3 "Natalia Gomez-Ospina | Stanford Medicine Profiles". med.stanford.edu. Retrieved 2021-01-23.
- 1 2 3 4 "Natalia Gomez-Ospina's Profile | Stanford Profiles". profiles.stanford.edu. Retrieved 2021-01-23.
- 1 2 University, © Stanford; Stanford; California 94305 (2018-11-02). "Natalia Gomez-Ospina - Assistant Professor of Pediatrics (Genetics and Stem Cell Transplantation)". Welcome to Bio-X. Retrieved 2021-01-23.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ↑ "Browsing by Author "Ospina Gómez, Natalia Andrea"". repositorio.unal.edu.co. Retrieved 2021-01-23.
- 1 2 "People". Gomez-Ospina Lab. Retrieved 2021-01-23.
- ↑ Fromherz, S. (2004-01-15). "Mutations in -tubulin promote basal body maturation and flagellar assembly in the absence of -tubulin". Journal of Cell Science. 117 (2): 303–314. doi: 10.1242/jcs.00859 . ISSN 0021-9533. PMID 14676280.
- ↑ "MSTP Alumni (before 2015)". MSTP MD-PhD Program. Retrieved 2021-01-23.
- ↑ "Dr. Natalia Gomez-Ospina, MD – Palo Alto, CA | Medical Genetics on Doximity". Doximity. Retrieved 2021-01-23.
- ↑ University, © Stanford; Stanford; California 94305 (2020-01-31). "Natalia Gomez-Ospina". Wu Tsai Neurosciences Institute. Retrieved 2021-01-23.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ↑ Gomez-Ospina, Natalia; Chang, Anne Lynn S.; Qu, Kun; Oro, Anthony E. (2012-06-07). "Translocation Affecting Sonic Hedgehog Genes in Basal-Cell Carcinoma". New England Journal of Medicine. 366 (23): 2233–2234. doi:10.1056/nejmc1115123. ISSN 0028-4793. PMC 3839666 . PMID 22670922.
- ↑ Gomez-Ospina, Natalia; Tsuruta, Fuminori; Barreto-Chang, Odmara; Hu, Linda; Dolmetsch, Ricardo (Nov 2006). "The C Terminus of the L-Type Voltage-Gated Calcium Channel CaV1.2 Encodes a Transcription Factor". Cell. 127 (3): 591–606. doi:10.1016/j.cell.2006.10.017. PMC 1750862 . PMID 17081980.
- ↑ Gomez-Ospina, Natalia; Potter, Carol J.; Xiao, Rui; Manickam, Kandamurugu; Kim, Mi-Sun; Kim, Kang Ho; Shneider, Benjamin L.; Picarsic, Jennifer L.; Jacobson, Theodora A.; Zhang, Jing; He, Weimin (Apr 2014). "Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis". Nature Communications. 7 (1): 10713. doi:10.1038/ncomms10713. ISSN 2041-1723. PMC 4759630 . PMID 26888176.
- ↑ Vakulskas, Christopher A.; Dever, Daniel P.; Rettig, Garrett R.; Turk, Rolf; Jacobi, Ashley M.; Collingwood, Michael A.; Bode, Nicole M.; McNeill, Matthew S.; Yan, Shuqi; Camarena, Joab; Lee, Ciaran M. (Aug 2018). "A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells". Nature Medicine. 24 (8): 1216–1224. doi:10.1038/s41591-018-0137-0. ISSN 1078-8956. PMC 6107069 . PMID 30082871.
- ↑ Charlesworth, Carsten T.; Deshpande, Priyanka S.; Dever, Daniel P.; Camarena, Joab; Lemgart, Viktor T.; Cromer, M. Kyle; Vakulskas, Christopher A.; Collingwood, Michael A.; Zhang, Liyang; Bode, Nicole M.; Behlke, Mark A. (Feb 2019). "Identification of preexisting adaptive immunity to Cas9 proteins in humans". Nature Medicine. 25 (2): 249–254. doi:10.1038/s41591-018-0326-x. ISSN 1078-8956. PMC 7199589 . PMID 30692695.
- ↑ Weiss, Karin; Terhal, Paulien A.; Cohen, Lior; Bruccoleri, Michael; Irving, Melita; Martinez, Ariel F.; Rosenfeld, Jill A.; Machol, Keren; Yang, Yaping; Liu, Pengfei; Walkiewicz, Magdalena (Oct 2016). "De Novo Mutations in CHD4, an ATP-Dependent Chromatin Remodeler Gene, Cause an Intellectual Disability Syndrome with Distinctive Dysmorphisms". The American Journal of Human Genetics. 99 (4): 934–941. doi:10.1016/j.ajhg.2016.08.001. PMC 5065651 . PMID 27616479.
- ↑ "Researchers develop a portable blood ammonia detector". EurekAlert!. Retrieved 2021-01-23.
- ↑ "Portable blood ammonia detector could be "life-changing"". New Atlas. 2020-07-22. Retrieved 2021-01-23.
- ↑ "Portable blood ammonia detector". ScienceDaily. Retrieved 2021-01-23.
- ↑ Kubota, Author Taylor (2020-07-24). "Device could help patients test blood ammonia levels at home". Scope. Retrieved 2021-01-23.
{{cite web}}:|first=has generic name (help)