
Trastuzumab, sold under the brand name Herceptin among others, is a monoclonal antibody used to treat breast cancer and stomach cancer. It is specifically used for cancer that is HER2 receptor positive. It may be used by itself or together with other chemotherapy medication. Trastuzumab is given by slow injection into a vein and injection just under the skin.
A cancer vaccine is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Receptor tyrosine-protein kinase erbB-2 is a protein that in humans is encoded by the ERBB2 gene. ERBB is abbreviated from erythroblastic oncogene B, a gene originally isolated from the avian genome. The human protein is also frequently referred to as HER2 or CD340.
Adjuvant therapy, also known as adjunct therapy, adjuvant care, or augmentation therapy, is a therapy that is given in addition to the primary or initial therapy to maximize its effectiveness. The surgeries and complex treatment regimens used in cancer therapy have led the term to be used mainly to describe adjuvant cancer treatments. An example of such adjuvant therapy is the additional treatment usually given after surgery where all detectable disease has been removed, but where there remains a statistical risk of relapse due to the presence of undetected disease. If known disease is left behind following surgery, then further treatment is not technically adjuvant.
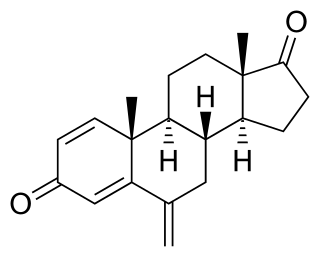
Exemestane, sold under the brand name Aromasin among others, is a medication used to treat breast cancer. It is a member of the class of antiestrogens known as aromatase inhibitors. Some breast cancers require estrogen to grow. Those cancers have estrogen receptors (ERs), and are called ER-positive. They may also be called estrogen-responsive, hormonally-responsive, or hormone-receptor-positive. Aromatase is an enzyme that synthesizes estrogen. Aromatase inhibitors block the synthesis of estrogen. This lowers the estrogen level, and slows the growth of cancers.
MammaPrint is a prognostic and predictive diagnostic test for early stage breast cancer patients that assess the risk that a tumor will metastasize to other parts of the body. It gives a binary result, high-risk or low-risk classification, and helps physicians determine whether or not a patient will benefit from chemotherapy. Women with a low risk result can safely forego chemotherapy without decreasing likelihood of disease free survival. MammaPrint is part of the personalized medicine portfolio marketed by Agendia.

Breast cancer chemotherapy refers to the use of cytotoxic drugs (chemotherapy) in the treatment of breast cancer.
Uschi Keszler's Pennies in Action Cancer Research Fund, holding a full 501(c)(3) non-profit foundation status, exists to support research for breast cancer curative programs, including preventative vaccines and other biological therapies that do not damage the immune system.

Uterine serous carcinoma is a malignant form of serous tumor that originates in the uterus. It is an uncommon form of endometrial cancer that typically arises in postmenopausal women. It is typically diagnosed on endometrial biopsy, prompted by post-menopausal bleeding.
Peptide-based synthetic vaccines, also called epitope vaccines, are subunit vaccines made from peptides. The peptides mimic the epitopes of the antigen that triggers direct or potent immune responses. Peptide vaccines can not only induce protection against infectious pathogens and non-infectious diseases but also be utilized as therapeutic cancer vaccines, where peptides from tumor-associated antigens are used to induce an effective anti-tumor T-cell response.
Neuvenge, Lapuleucel-T, is a therapeutic cancer vaccine (TCV) in development by Dendreon (DNDN). It uses the "immunotherapy platform approach" first successfully demonstrated on the U.S. Food and Drug Administration (FDA)-approved TCV Provenge. It was first tested on breast cancer patients with tumors expressing HER2/neu, and is now scheduled to be tested on bladder cancer patients.

Trastuzumab emtansine, sold under the brand name Kadcyla, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the cytotoxic agent DM1. Trastuzumab alone stops growth of cancer cells by binding to the HER2 receptor, whereas trastuzumab emtansine undergoes receptor-mediated internalization into cells, is catabolized in lysosomes where DM1-containing catabolites are released and subsequently bind tubulin to cause mitotic arrest and cell death. Trastuzumab binding to HER2 prevents homodimerization or heterodimerization (HER2/HER3) of the receptor, ultimately inhibiting the activation of MAPK and PI3K/AKT cellular signalling pathways. Because the monoclonal antibody targets HER2, and HER2 is only over-expressed in cancer cells, the conjugate delivers the cytotoxic agent DM1 specifically to tumor cells. The conjugate is abbreviated T-DM1.
CimaVax-EGF is a vaccine used to treat cancer, specifically non-small-cell lung carcinoma (NSCLC). CIMAvax-EGF is composed of recombinant human epidermal growth factor (EGF) conjugated to a protein carrier.

Galena Biopharma was a publicly traded pharmaceutical company based in San Ramon, California. The company was founded in Worcester, Massachusetts. In 2011, it moved to Oregon, and in 2015 moved to San Ramon, California. Mark Schwartz was the company's president and chief executive officer. As of December 29, 2017, the company was acquired by Sellas Life Sciences Group Ltd. through a reverse merger transaction. Galena Biopharma was renamed to Sellas Life Sciences Group, Inc.
Eftilagimod alpha is a large-molecule cancer drug being developed by the clinical-stage biotechnology company Immutep. Efti is a soluble version of the immune checkpoint molecule LAG-3. It is an APC Activator used to increase an immune response to tumors, and is administered by subcutaneous injection. Efti has three intended clinical settings:
Kathleen I. Pritchard, is the head of oncology at Sunnybrook Health Sciences Centre in Toronto, Canada, specializing in breast cancer therapies, and leading the clinical trials division of the centre. She has authored numerous studies on women's health, breast cancer, hormone replacement therapy, public health, and research methodology. According to Thomson Reuters, Pritchard was one of the most cited researchers in the world in 2014 and 2015.
Imugene Ltd is a biotechnology company working in cancer immunotherapy. The company's lead product is HER-Vaxx, a therapeutic cancer vaccine for the treatment of gastric cancer and breast cancer, where the cancer is HER-2-positive. Imugene was planning a Phase Ib/II clinical study of HER-Vaxx in gastric cancer which it intended to initiate in 2016. Imugene stock is publicly traded on the Australian Securities Exchange (ASX).
Vaccinogen Inc. is a US biotechnology company based in Baltimore. It is currently developing a potential cancer immunotherapy called OncoVAX, where a patient’s own tumor cells are used as the vaccine, adjuvanted by BCG. This product was evaluated in Phase III in colon cancer in the 1990s and another Phase III study, called ACTIVE, is currently recruiting stage II colon cancer patients. Vaccinogen calls its approach 'Active Specific Immunotherapy' or ASI.
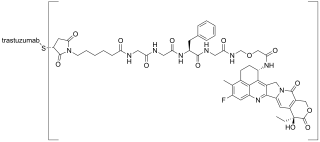
Trastuzumab deruxtecan, sold under the brand name Enhertu, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the topoisomerase I inhibitor deruxtecan. It is licensed for the treatment of breast cancer or gastric or gastroesophageal adenocarcinoma. Trastuzumab binds to and blocks signaling through epidermal growth factor receptor 2 (HER2/neu) on cancers that rely on it for growth. Additionally, once bound to HER2 receptors, the antibody is internalized by the cell, carrying the bound deruxtecan along with it, where it interferes with the cell's ability to make DNA structural changes and replicate its DNA during cell division, leading to DNA damage when the cell attempts to replicate itself, destroying the cell.
Trastuzumab/hyaluronidase, sold under the brand name Herceptin SC among others, is a fixed-dose combination medication for the treatment of HER2-overexpressing breast cancer in adults. It is a combination of trastuzumab and hyaluronidase.







