Related Research Articles
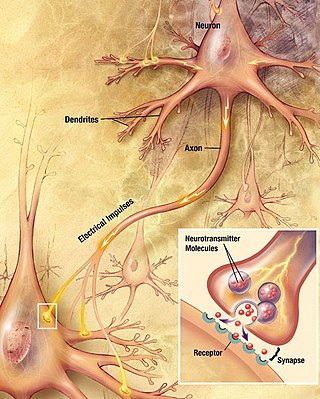
Chemical synapses are biological junctions through which neurons' signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous system. They are crucial to the biological computations that underlie perception and thought. They allow the nervous system to connect to and control other systems of the body.

In neuroscience, long-term potentiation (LTP) is a persistent strengthening of synapses based on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between two neurons. The opposite of LTP is long-term depression, which produces a long-lasting decrease in synaptic strength.
In neuroscience, synaptic plasticity is the ability of synapses to strengthen or weaken over time, in response to increases or decreases in their activity. Since memories are postulated to be represented by vastly interconnected neural circuits in the brain, synaptic plasticity is one of the important neurochemical foundations of learning and memory.

In neuroscience, an excitatory postsynaptic potential (EPSP) is a postsynaptic potential that makes the postsynaptic neuron more likely to fire an action potential. This temporary depolarization of postsynaptic membrane potential, caused by the flow of positively charged ions into the postsynaptic cell, is a result of opening ligand-gated ion channels. These are the opposite of inhibitory postsynaptic potentials (IPSPs), which usually result from the flow of negative ions into the cell or positive ions out of the cell. EPSPs can also result from a decrease in outgoing positive charges, while IPSPs are sometimes caused by an increase in positive charge outflow. The flow of ions that causes an EPSP is an excitatory postsynaptic current (EPSC).
In neurophysiology, long-term depression (LTD) is an activity-dependent reduction in the efficacy of neuronal synapses lasting hours or longer following a long patterned stimulus. LTD occurs in many areas of the CNS with varying mechanisms depending upon brain region and developmental progress.
In neuroscience, a silent synapse is an excitatory glutamatergic synapse whose postsynaptic membrane contains NMDA-type glutamate receptors but no AMPA-type glutamate receptors. These synapses are named "silent" because normal AMPA receptor-mediated signaling is not present, rendering the synapse inactive under typical conditions. Silent synapses are typically considered to be immature glutamatergic synapses. As the brain matures, the relative number of silent synapses decreases. However, recent research on hippocampal silent synapses shows that while they may indeed be a developmental landmark in the formation of a synapse, that synapses can be "silenced" by activity, even once they have acquired AMPA receptors. Thus, silence may be a state that synapses can visit many times during their lifetimes.

In a neuron, synaptic vesicles store various neurotransmitters that are released at the synapse. The release is regulated by a voltage-dependent calcium channel. Vesicles are essential for propagating nerve impulses between neurons and are constantly recreated by the cell. The area in the axon that holds groups of vesicles is an axon terminal or "terminal bouton". Up to 130 vesicles can be released per bouton over a ten-minute period of stimulation at 0.2 Hz. In the visual cortex of the human brain, synaptic vesicles have an average diameter of 39.5 nanometers (nm) with a standard deviation of 5.1 nm.
Schaffer collaterals are axon collaterals given off by CA3 pyramidal cells in the hippocampus. These collaterals project to area CA1 of the hippocampus and are an integral part of memory formation and the emotional network of the Papez circuit, and of the hippocampal trisynaptic loop. It is one of the most studied synapses in the world and named after the Hungarian anatomist-neurologist Károly Schaffer.
Metaplasticity is a term originally coined by W.C. Abraham and M.F. Bear to refer to the plasticity of synaptic plasticity. Until that time synaptic plasticity had referred to the plastic nature of individual synapses. However this new form referred to the plasticity of the plasticity itself, thus the term meta-plasticity. The idea is that the synapse's previous history of activity determines its current plasticity. This may play a role in some of the underlying mechanisms thought to be important in memory and learning such as long-term potentiation (LTP), long-term depression (LTD) and so forth. These mechanisms depend on current synaptic "state", as set by ongoing extrinsic influences such as the level of synaptic inhibition, the activity of modulatory afferents such as catecholamines, and the pool of hormones affecting the synapses under study. Recently, it has become clear that the prior history of synaptic activity is an additional variable that influences the synaptic state, and thereby the degree, of LTP or LTD produced by a given experimental protocol. In a sense, then, synaptic plasticity is governed by an activity-dependent plasticity of the synaptic state; such plasticity of synaptic plasticity has been termed metaplasticity. There is little known about metaplasticity, and there is much research currently underway on the subject, despite its difficulty of study, because of its theoretical importance in brain and cognitive science. Most research of this type is done via cultured hippocampus cells or hippocampal slices.

In the nervous system, a synapse is a structure that permits a neuron to pass an electrical or chemical signal to another neuron or to the target effector cell.

Synaptic potential refers to the potential difference across the postsynaptic membrane that results from the action of neurotransmitters at a neuronal synapse. In other words, it is the “incoming” signal that a neuron receives. There are two forms of synaptic potential: excitatory and inhibitory. The type of potential produced depends on both the postsynaptic receptor, more specifically the changes in conductance of ion channels in the post synaptic membrane, and the nature of the released neurotransmitter. Excitatory post-synaptic potentials (EPSPs) depolarize the membrane and move the potential closer to the threshold for an action potential to be generated. Inhibitory postsynaptic potentials (IPSPs) hyperpolarize the membrane and move the potential farther away from the threshold, decreasing the likelihood of an action potential occurring. The Excitatory Post Synaptic potential is most likely going to be carried out by the neurotransmitters glutamate and acetylcholine, while the Inhibitory post synaptic potential will most likely be carried out by the neurotransmitters gamma-aminobutyric acid (GABA) and glycine. In order to depolarize a neuron enough to cause an action potential, there must be enough EPSPs to both depolarize the postsynaptic membrane from its resting membrane potential to its threshold and counterbalance the concurrent IPSPs that hyperpolarize the membrane. As an example, consider a neuron with a resting membrane potential of -70 mV (millivolts) and a threshold of -50 mV. It will need to be raised 20 mV in order to pass the threshold and fire an action potential. The neuron will account for all the many incoming excitatory and inhibitory signals via summative neural integration, and if the result is an increase of 20 mV or more, an action potential will occur.
Augmentation is one of four components of short-term synaptic plasticity that increases the probability of releasing synaptic vesicles during and after repetitive stimulation such that

Summation, which includes both spatial summation and temporal summation, is the process that determines whether or not an action potential will be generated by the combined effects of excitatory and inhibitory signals, both from multiple simultaneous inputs, and from repeated inputs. Depending on the sum total of many individual inputs, summation may or may not reach the threshold voltage to trigger an action potential.

Axon terminals are distal terminations of the branches of an axon. An axon, also called a nerve fiber, is a long, slender projection of a nerve cell that conducts electrical impulses called action potentials away from the neuron's cell body in order to transmit those impulses to other neurons, muscle cells or glands. In the central nervous system, most presynaptic terminals are actually formed along the axons, not at their ends.
Cellular neuroscience is a branch of neuroscience concerned with the study of neurons at a cellular level. This includes morphology and physiological properties of single neurons. Several techniques such as intracellular recording, patch-clamp, and voltage-clamp technique, pharmacology, confocal imaging, molecular biology, two photon laser scanning microscopy and Ca2+ imaging have been used to study activity at the cellular level. Cellular neuroscience examines the various types of neurons, the functions of different neurons, the influence of neurons upon each other, and how neurons work together.

Nonsynaptic plasticity is a form of neuroplasticity that involves modification of ion channel function in the axon, dendrites, and cell body that results in specific changes in the integration of excitatory postsynaptic potentials and inhibitory postsynaptic potentials. Nonsynaptic plasticity is a modification of the intrinsic excitability of the neuron. It interacts with synaptic plasticity, but it is considered a separate entity from synaptic plasticity. Intrinsic modification of the electrical properties of neurons plays a role in many aspects of plasticity from homeostatic plasticity to learning and memory itself. Nonsynaptic plasticity affects synaptic integration, subthreshold propagation, spike generation, and other fundamental mechanisms of neurons at the cellular level. These individual neuronal alterations can result in changes in higher brain function, especially learning and memory. However, as an emerging field in neuroscience, much of the knowledge about nonsynaptic plasticity is uncertain and still requires further investigation to better define its role in brain function and behavior.
Post-tetanic potentiation (PTP) is a form of synaptic plasticity which is short-lived and results in increased frequency of miniature excitatory postsynaptic potentials (mEPSPs) or currents (EPSCs) with no effect on amplitude in the spontaneous postsynaptic potential. It usually lasts in the range of several minutes. PTPs are observed when synapses are stimulated with repetitive (tetanic) pulses, by means of prolonged trains of stimuli applied at high frequencies.

The active zone or synaptic active zone is a term first used by Couteaux and Pecot-Dechavassinein in 1970 to define the site of neurotransmitter release. Two neurons make near contact through structures called synapses allowing them to communicate with each other. As shown in the adjacent diagram, a synapse consists of the presynaptic bouton of one neuron which stores vesicles containing neurotransmitter, and a second, postsynaptic neuron which bears receptors for the neurotransmitter, together with a gap between the two called the synaptic cleft. When an action potential reaches the presynaptic bouton, the contents of the vesicles are released into the synaptic cleft and the released neurotransmitter travels across the cleft to the postsynaptic neuron and activates the receptors on the postsynaptic membrane.
In neuroscience, synaptic scaling is a form of homeostatic plasticity, in which the brain responds to chronically elevated activity in a neural circuit with negative feedback, allowing individual neurons to reduce their overall action potential firing rate. Where Hebbian plasticity mechanisms modify neural synaptic connections selectively, synaptic scaling normalizes all neural synaptic connections by decreasing the strength of each synapse by the same factor, so that the relative synaptic weighting of each synapse is preserved.

Synaptic fatigue, or short-term synaptic depression, is an activity-dependent form of short term synaptic plasticity that results in the temporary inability of neurons to fire and therefore transmit an input signal. It is thought to be a form of negative feedback in order to physiologically control particular forms of nervous system activity.
References
- 1 2 3 Zucker, Robert S.; Regehr, Wade G. (2002). "Short-Term Synaptic Plasticity". Annu. Rev. Physiol. 64: 355–405. doi:10.1146/annurev.physiol.64.092501.114547. PMID 11826273. S2CID 7980969.
- ↑ Fortune, Eric S.; Rose, Gary J. (2001). "Short-term synaptic plasticity as a temporal filter". Trends in Neurosciences. 24 (7): 381–5. doi:10.1016/s0166-2236(00)01835-x. PMID 11410267. S2CID 14642561.
- 1 2 3 4 Abbot, LF; Regehr, WG (2004). "Synaptic Computation". Nature. 431 (7010): 796–803. Bibcode:2004Natur.431..796A. doi:10.1038/nature03010. PMID 15483601. S2CID 2075305.
- 1 2 Purves, Dale; Augustine, George J.; Fitzpatrick, David; Hall, William C.; LaMantia, Anthony-Samuel; While, Leonard E. (2012). Neuroscience (5th ed.). Sunderland, MA: Sinauer. ISBN 978-0-87893-695-3.
- ↑ Del Castillo, J; Katz, B (1954). "Statistical factors involved in neuromuscular facilitation and depression". J. Physiol. 124 (3): 574–585. doi:10.1113/jphysiol.1954.sp005130. PMC 1366293 . PMID 13175200.
- ↑ Dudel, J; Kuffler, SW (1961). "Mechanism of facilitation at the crayfish neuromuscular junction". J. Physiol. 155 (3): 530–542. doi:10.1113/jphysiol.1961.sp006645. PMC 1359873 . PMID 13724751.
- ↑ Katz, B; Miledi, R (1968), "The role of calcium in neuromuscular facilitation", J. Physiol., 195 (2): 481–492, doi:10.1113/jphysiol.1968.sp008469, PMC 1351674 , PMID 4296699
- 1 2 Jianhua, Xu; Liming, He; Ling-Gang, Wu (2007), "Role of Ca2+ channels in short-term synaptic plasticity", Current Opinion in Neurobiology, 17 (3): 352–9, doi:10.1016/j.conb.2007.04.005, PMID 17466513, S2CID 140207065
- ↑ Díaz-Rojas, Françoise; Sakaba, Takeshi; Kawaguchi, Shin-Ya (Nov 15, 2015). "Ca(2+) current facilitation determines short-term facilitation at inhibitory synapses between cerebellar Purkinje cells". Journal of Physiology. 593 (22): 4889–904. doi:10.1113/JP270704. PMC 4650412 . PMID 26337248.
- ↑ Thomson, Alex M. (2000). "Facilitation augmentation and potentiation at central synapses". Trends in Neurosciences. 23 (7): 305–312. doi:10.1016/s0166-2236(00)01580-0. PMID 10856940. S2CID 14758903.
- ↑ Prescott, Steven (May 2012). "Interactions between Depression and Facilitation within Neural Networks: Updating the Dual-Process Theory of Plasticity". Learning & Memory. 19 (5): 446–466. doi: 10.1101/lm.5.6.446 . PMC 311261 . PMID 10489261. S2CID 20399342.
- 1 2 MacLeod, KM (2011). "Short-term synaptic plasticity and intensity coding". Hearing Research. 279 (1–2): 13–21. doi:10.1016/j.heares.2011.03.001. PMC 3210195 . PMID 21397676.