Saltpeter (or saltpetre) is the mineral form of potassium nitrate (KNO3), a compound

Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula KNO
3. It is an ionic salt of potassium ions K+ and nitrate ions NO3−, and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or nitre in the UK). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpeter (or saltpetre in the UK).

Natron is a naturally occurring mixture of sodium carbonate decahydrate (Na2CO3·10H2O, a kind of soda ash) and around 17% sodium bicarbonate (also called baking soda, NaHCO3) along with small quantities of sodium chloride and sodium sulfate. Natron is white to colourless when pure, varying to gray or yellow with impurities. Natron deposits are sometimes found in saline lake beds which arose in arid environments. Throughout history natron has had many practical applications that continue today in the wide range of modern uses of its constituent mineral components.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the Chlor-alkali process.
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities are on the right-hand side with a plus sign between the entities in both the reactants and the products, and an arrow that points towards the products to show the direction of the reaction. The chemical formulas may be symbolic, structural, or intermixed. The coefficients next to the symbols and formulas of entities are the absolute values of the stoichiometric numbers. The first chemical equation was diagrammed by Jean Beguin in 1615.

Sodium nitrate is the chemical compound with the formula NaNO
3. This alkali metal nitrate salt is also known as Chile saltpeter to distinguish it from ordinary saltpeter, potassium nitrate. The mineral form is also known as nitratine, nitratite or soda niter.
The nitrite ion has the chemical formula NO−
2. Nitrite is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid.

Niter or nitre is the mineral form of potassium nitrate, KNO3. It is a soft, white, highly soluble mineral found primarily in arid climates or cave deposits.
The nitrophosphate process is a method for the industrial production of nitrogen fertilizers invented by Erling Johnson in the municipality of Odda, Norway around 1927.

Thermonatrite is a naturally occurring evaporite mineral form of sodium carbonate, Na2CO3·H2O.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.
The sodium fusion test, or Lassaigne's test, is used in elemental analysis for the qualitative determination of the presence of foreign elements, namely halogens, nitrogen, and sulfur, in an organic compound. It was developed by J. L. Lassaigne.

Magnesium nitrate refers to inorganic compounds with the formula Mg(NO3)2(H2O)x, where x = 6, 2, and 0. All are white solids. The anhydrous material is hygroscopic, quickly forming the hexahydrate upon standing in air. All of the salts are very soluble in both water and ethanol.
Sodium oxide is a chemical compound with the formula Na2O. It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to describe components of various materials such as glasses and fertilizers which contain oxides that include sodium and other elements. Sodium oxide is a component.
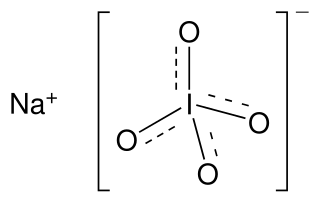
Sodium periodate is an inorganic salt, composed of a sodium cation and the periodate anion. It may also be regarded as the sodium salt of periodic acid. Like many periodates, it can exist in two different forms: sodium metaperiodate (formula NaIO4) and sodium orthoperiodate (normally Na2H3IO6, but sometimes the fully reacted salt Na5IO6). Both salts are useful oxidising agents.

Carbonate minerals are those minerals containing the carbonate ion, CO2−
3.

Sodium hexanitritocobaltate(III) is inorganic compound with the formula Na3[Co(NO2)6]. The anion of this yellow-coloured salt consists of the transition metal nitrite complex [Co(NO2)6]3−. It was a reagent for the qualitative test for potassium and ammonium ions.

Curing salt is used in meat processing to generate a pinkish shade and to extend shelf life. It is both a color agent and a means to facilitate food preservation as it prevents or slows spoilage by bacteria or fungus. Curing salts are generally a mixture of sodium chloride and sodium nitrite, and are used for pickling meats as part of the process to make sausage or cured meat such as ham, bacon, pastrami, corned beef, etc. Though it has been suggested that the reason for using nitrite-containing curing salt is to prevent botulism, a 2018 study by the British Meat Producers Association determined that legally permitted levels of nitrite have no effect on the growth of the Clostridium botulinum bacteria that causes botulism, in line with the UK's Advisory Committee on the Microbiological Safety of Food opinion that nitrites are not required to prevent C. botulinum growth and extend shelf life..
Orthonitrate is a tetrahedral anion of nitrogen with the formula NO3−
4. It was first identified in 1977 and is currently known in only two compounds, sodium orthonitrate (Na3NO4) and potassium orthonitrate (K3NO4). The corresponding oxoacid, orthonitric acid (H3NO4), is hypothetical and has never been observed. Sodium and potassium orthonitrate can be prepared by fusion of the nitrate and metal oxide under high temperatures and ideally high pressures (several GPa).
The sulfate nitrates are a family of double salts that contain both sulfate and nitrate ions (NO3−, SO42−). They are in the class of mixed anion compounds. A few rare minerals are in this class. Two sulfate nitrates are in the class of anthropogenic compounds, accidentally made as a result of human activities in fertilizers that are a mix of ammonium nitrate and ammonium sulfate, and also in the atmosphere as polluting ammonia, nitrogen dioxide, and sulfur dioxide react with the oxygen and water there to form solid particles. The nitro group (NO3−) can act as a ligand, and complexes containing it can form salts with sulfate.


















