Function
Nod factors are potentially recognized by plant receptors made of two histidine kinases with extracellular LysM domain, which have been identified in L. japonicus, soybean, and M. truncatula [5] . Binding of Nod factors to these receptors depolarizes the plasma membrane of root hairs via an influx of Ca+2 which induce the expression of early nodulin (ENOD) genes and swelling of the root hairs. In M. truncatula, the signal transduction initiates by the activation of dmi1, dmi2, and dmi3 which lead to the deformation of root hairs, early nodulin expression, cortical cell division and bacterial infection. Additionally, nsp and hcl genes are recruited later and aid in the process of early nodulation expression, cortical cell division, and infection. [6] Genes dmi1, dmi2, and dmi3 have also been found to aid in the establishment of interactions between M. truncatula and arbuscular mycorrhiza, indicating that the two very different symbioses may share some common mechanisms. [7] The end result is the nodule, the structure in which nitrogen is fixed. Nod factors act by inducing changes in gene expression in the legume, most notable the nodulin genes, which are needed for nodule organogenesis. [8]
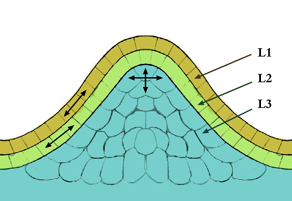
In cell biology, the meristem is a type of tissue found in plants. It consists of undifferentiated cells capable of cell division. Cells in the meristem can develop into all the other tissues and organs that occur in plants. These cells continue to divide until they become differentiated and lose the ability to divide.

Rhizobia are diazotrophic bacteria that fix nitrogen after becoming established inside the root nodules of legumes (Fabaceae). To express genes for nitrogen fixation, rhizobia require a plant host; they cannot independently fix nitrogen. In general, they are gram negative, motile, non-sporulating rods.

Medicago truncatula, the barrelclover, strong-spined medick, barrel medic, or barrel medick, is a small annual legume native to the Mediterranean region that is used in genomic research. It is a low-growing, clover-like plant 10–60 centimetres (3.9–23.6 in) tall with trifoliate leaves. Each leaflet is rounded, 1–2 centimetres (0.39–0.79 in) long, often with a dark spot in the center. The flowers are yellow, produced singly or in a small inflorescence of two to five together; the fruit is a small, spiny pod.

Ensifer meliloti are an aerobic, Gram-negative, and diazotrophic species of bacteria. S. meliloti are motile and possess a cluster of peritrichous flagella. S. meliloti fix atmospheric nitrogen into ammonia for their legume hosts, such as alfalfa. S. meliloti forms a symbiotic relationship with legumes from the genera Medicago, Melilotus and Trigonella, including the model legume Medicago truncatula. This symbiosis promotes the development of a plant organ, termed a root nodule. Because soil often contains a limited amount of nitrogen for plant use, the symbiotic relationship between S. meliloti and their legume hosts has agricultural applications. These techniques reduce the need for inorganic nitrogenous fertilizers.

Root nodules are found on the roots of plants, primarily legumes, that form a symbiosis with nitrogen-fixing bacteria. Under nitrogen-limiting conditions, capable plants form a symbiotic relationship with a host-specific strain of bacteria known as rhizobia. This process has evolved multiple times within the legumes, as well as in other species found within the Rosid clade. Legume crops include beans, peas, and soybeans.
Sharon Rugel Long is an American plant biologist. She is the Steere-Pfizer Professor of Biological Science in the Department of Biology at Stanford University, and the Principal Investigator of the Long Laboratory at Stanford.
Symbiotic bacteria are bacteria living in symbiosis with another organism or each other. For example, rhizobia living in root nodules of legumes provide nitrogen fixing activity for these plants.
Horizontal transmission is the transmission of organisms between biotic and/or abiotic members of an ecosystem that are not in a parent-progeny relationship. Because the evolutionary fate of the agent is not tied to reproductive success of the host, horizontal transmission tends to evolve virulence. It is therefore a critical concept for evolutionary medicine.

Bradyrhizobium is a genus of Gram-negative soil bacteria, many of which fix nitrogen. Nitrogen fixation is an important part of the nitrogen cycle. Plants cannot use atmospheric nitrogen (N2); they must use nitrogen compounds such as nitrates.
The nif genes are genes encoding enzymes involved in the fixation of atmospheric nitrogen into a form of nitrogen available to living organisms. The primary enzyme encoded by the nif genes is the nitrogenase complex which is in charge of converting atmospheric nitrogen (N2) to other nitrogen forms such as ammonia which the organism can use for various purposes. Besides the nitrogenase enzyme, the nif genes also encode a number of regulatory proteins involved in nitrogen fixation. The nif genes are found in both free-living nitrogen-fixing bacteria and in symbiotic bacteria associated with various plants. The expression of the nif genes is induced as a response to low concentrations of fixed nitrogen and oxygen concentrations (the low oxygen concentrations are actively maintained in the root environment of host plants). The first Rhizobium genes for nitrogen fixation (nif) and for nodulation (nod) were cloned in the early 1980s by Gary Ruvkun and Sharon R. Long in Frederick M. Ausubel's laboratory.
Actinorhizal plants are a group of angiosperms characterized by their ability to form a symbiosis with the nitrogen fixing actinomycetota Frankia. This association leads to the formation of nitrogen-fixing root nodules.

enod40, also known as early nodulin 40, is a gene found in flowering plants. The gene has characteristics of both protein and Non-coding RNA genes. There is some evidence that the non-coding characteristics of this gene are more widely conserved than the protein coding sequences. In soyabeans enod40 was found to be expressed during early stages of formation of nitrogen-fixing root nodules that are associated with symbiotic soil rhizobial bacteria. The gene is also active in roots containing fungi forming phosphate-acquiring arbuscular mycorrhiza. An interaction with a novel RNA-binding protein MtRBP1 investigated in the development of Root nodule suggests ENOD40 has a function of cytoplasmic relocalization of nuclear proteins. In the study of non-legume plants, the over-expression of ENOD40 in transgenic Arabidopsis lines was observed a reduction of cell expansion.

Within genetics, post-genomic research has rendered bacterial small non-coding RNAs (sRNAs) as major players in post-transcriptional regulation of gene expression in response to environmental stimuli. The Alphaproteobacteria includes Gram-negative microorganisms with diverse life styles; frequently involving long-term interactions with higher eukaryotes.
Ensifer medicae is a species of gram-negative, nitrogen-fixing, rod-shaped bacteria. They can be free-living or symbionts of leguminous plants in root nodules. E.medicae was first isolated from root nodules on plants in the genus Medicago. Some strains of E.medicae, like WSM419, are aerobic. They are chemoorganotrophic mesophiles that prefer temperatures around 28 °C. In addition to their primary genome, these organisms also have three known plasmids, sized 1,570,951 bp, 1,245,408 bp and 219,313 bp.
Martin Parniske is a German biologist with a specialisation in genetics, microbiology and biochemistry. He is university professor and head of the Institute of Genetics at the Faculty of Biology of the Ludwig Maximilian University of Munich. Parniske's scientific focus is on the molecular interaction between plants and symbiotic and pathogenic organisms including bacteria, fungi, oomycetes and insects.

In molecular biology the LysM domain is a protein domain found in a wide variety of extracellular proteins and receptors. The LysM domain is named after the Lysin Motif which was the original name given to the sequence motif identified in bacterial proteins. The region was originally identified as a C-terminal repeat found in the Enterococcus hirae muramidase. The LysM domain is found in a wide range of microbial extracellular proteins, where the LysM domain is thought to provide an anchoring to extracellular polysaccharides such as peptidoglycan and chitin. LysM domains are also found in plant receptors, including NFP, the receptor for Nod factor which is necessary for the root nodule symbiosis between legumes and symbiotic bacteria. The LysM domain is typically between 44 and 65 amino acid residues in length. The structure of the LysM domain showed that it is composed of a pair of antiparallel beta strands separated by a pair of short alpha helices.

A symbiosome is a specialised compartment in a host cell that houses an endosymbiont in a symbiotic relationship.
Myriam Charpentier is a molecular biologist, who specialises in cell and developmental biology at the John Innes Centre, Norwich. Charpentier studies the environmental and biological stimulus of nuclear calcium signalling in plants.
Plant nucleus movement is the movement of the cell nucleus in plants by the cytoskeleton.

The common symbiosis signaling pathway (CSSP) is a signaling cascade in plants that allows them to interact with symbiotic microbes. It corresponds to an ancestral pathway that plants use to interact with arbuscular mycorrhizal fungi (AMF). It is known as "common" because different evolutionary younger symbioses also use this pathway, notably the root nodule symbiosis with nitrogen-fixing rhizobia bacteria. The pathway is activated by both Nod-factor perception, as well as by Myc-factor perception that are released from AMF. The pathway is distinguished from the pathogen recognition pathways, but may have some common receptors involved in both pathogen recognition as well as CSSP. A recent work by Kevin Cope and colleagues showed that ectomycorrhizae also uses CSSP components such as Myc-factor recognition.









