
Monoamine oxidases (MAO) are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases.

A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.

Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, serotonin and epinephrine.
A neurochemical is a small organic molecule or peptide that participates in neural activity. The science of neurochemistry studies the functions of neurochemicals.
The vesicular monoamine transporter (VMAT) is a transport protein integrated into the membrane of synaptic vesicles of presynaptic neurons. It acts to transport monoamine neurotransmitters – such as dopamine, serotonin, norepinephrine, epinephrine, and histamine – into the vesicles, which release the neurotransmitters into synapses as chemical messages to postsynaptic neurons. VMATs utilize a proton gradient generated by V-ATPases in vesicle membranes to power monoamine import.

Vesicular monoamine transporter 1 (VMAT1) also known as chromaffin granule amine transporter (CGAT) or solute carrier family 18 member 1 (SLC18A1) is a protein that in humans is encoded by the SLC18A1 gene. VMAT1 is an integral membrane protein, which is embedded in synaptic vesicles and serves to transfer monoamines, such as norepinephrine, epinephrine, dopamine, and serotonin, between the cytosol and synaptic vesicles. SLC18A1 is an isoform of the vesicular monoamine transporter.

Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as a hormone and neurotransmitter. The name "noradrenaline", derived from Latin roots meaning "at/alongside the kidneys", is more commonly used in the United Kingdom; in the United States, "norepinephrine", derived from Greek roots having that same meaning, is usually preferred. "Norepinephrine" is also the international nonproprietary name given to the drug. Regardless of which name is used for the substance itself, parts of the body that produce or are affected by it are referred to as noradrenergic.

Trace amine-associated receptor 1 (TAAR1) is a trace amine-associated receptor (TAAR) protein that in humans is encoded by the TAAR1 gene. TAAR1 is an intracellular amine-activated Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) that is primarily expressed in several peripheral organs and cells, astrocytes, and in the intracellular milieu within the presynaptic plasma membrane of monoamine neurons in the central nervous system (CNS). TAAR1 was discovered in 2001 by two independent groups of investigators, Borowski et al. and Bunzow et al. TAAR1 is one of six functional human trace amine-associated receptors, which are so named for their ability to bind endogenous amines that occur in tissues at trace concentrations. TAAR1 plays a significant role in regulating neurotransmission in dopamine, norepinephrine, and serotonin neurons in the CNS; it also affects immune system and neuroimmune system function through different mechanisms.

The plasma membrane monoamine transporter (PMAT) is a low-affinity monoamine transporter protein which in humans is encoded by the SLC29A4 gene. It is known alternatively as the human equilibrative nucleoside transporter-4 (hENT4). Unlike other members of the ENT family, it is impermeable to most nucleosides, with the exception of the inhibitory neurotransmitter and ribonucleoside adenosine, which it is permeable to in a highly pH-dependent manner.

Amiflamine (FLA-336) is a reversible inhibitor of monoamine oxidase A (MAO-A), thereby being a RIMA, and, to a lesser extent, semicarbazide-sensitive amine oxidase (SSAO), as well as a serotonin releasing agent (SRA). It is a derivative of the phenethylamine and amphetamine chemical classes. The (+)-enantiomer is the active stereoisomer.
Noradrenergic cell group A1 is a group of cells in the vicinity of the lateral reticular nucleus of the medullary reticular formation that label for norepinephrine in primates and rodents. They are found in the ventrolateral medulla in conjunction with the adrenergic cell group C1.
Noradrenergic cell group A2 is a group of cells in the vicinity of the dorsal motor nucleus of the vagus nerve in the medulla that label for norepinephrine in primates and rodents.
Noradrenergic cell group A4 is a group of cells exhibiting noradrenergic fluorescence that, in the rat, are located in the Tegmen ventriculi quarti ventral to the cerebellar nuclei, and in the macaque, are found at the edge of the lateral recess of the fourth ventricle caudally, extending to beneath the floor of the ventricle where they merge with the noradrenergic group A6, the locus ceruleus.
Noradrenergic cell group A5 is a group of cells in the vicinity of the superior olivary complex in the pontine tegmentum that label for norepinephine in primates, rodents and other mammals.
Noradrenergic cell group A6sc is a group of cells fluorescent for norepinephrine that are scattered in the nucleus subceruleus of the macaque.,
Noradrenergic cell group Acg is a group of cells fluorescent for norepinephrine that are located in the central gray of the midbrain at the level of the trochlear nucleus in the squirrel monkey (Saimiri) and to a lesser degree in the macaque.
Dopaminergic cell groups are collections of neurons in the central nervous system that synthesize the neurotransmitter dopamine. In the 1960s, dopamine neurons were first identified and named by Annica Dahlström and sv:Kjell Fuxe, who used histochemical fluorescence. The subsequent discovery of genes encoding enzymes that synthesize dopamine, and transporters that incorporate dopamine into synaptic vesicles or reclaim it after synaptic release, enabled scientists to identify dopaminergic neurons by labeling gene or protein expression that is specific to these neurons.
Serotonergic cell groups refer to collections of neurons in the central nervous system that have been demonstrated by histochemical fluorescence to contain the neurotransmitter serotonin (5-hydroxytryptamine). Since they are for the most part localized to classical brainstem nuclei, particularly the raphe nuclei, they are more often referred to by the names of those nuclei than by the B1-9 nomenclature. These cells appear to be common across most mammals and have two main regions in which they develop; one forms in the mesencephlon and the rostral pons and the other in the medulla oblongata and the caudal pons.
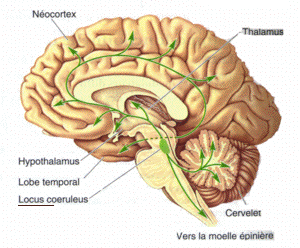
Monoamine nuclei are clusters of cells that primarily use monoamine neurotransmitters to communicate. The raphe nuclei, ventral tegmental area, and locus coeruleus have been included in texts about monoamine nuclei. These nuclei receive a variety of inputs including from other monoamines, as well as from glutaminergic, GABAergic, and substance p related pathways. The catacholaminergic pathways mainly project upwards into the cortical and limbic regions, power sparse descending axons have been observed in animals models. Both ascending and descending serotonergic pathways project from the raphe nuclei. Raphe nuclei in the obscurus, pallid us, and magnus descend into the brainstem and spinal cord, while the raphe ponds, raphe dorsals, and nucleus centralism superior projected up into the medial forebrain bundle before branching off. Monoamine nucleic have been studied in relation to major depressive disorder, with some abnormalities observed, however MAO-B levels appear to be normal during depression in these regions.









