Related Research Articles
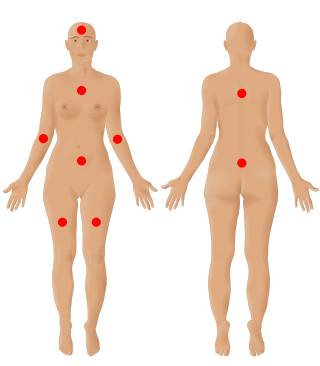
Fibromyalgia is a medical condition defined by the presence of chronic widespread pain, fatigue, waking unrefreshed, cognitive symptoms, lower abdominal pain or cramps, and depression. Other symptoms include insomnia and a general hypersensitivity.

Transcutaneous electrical nerve stimulation is the use of electric current produced by a device to stimulate the nerves for therapeutic purposes. TENS, by definition, covers the complete range of transcutaneously applied currents used for nerve excitation, but the term is often used with a more restrictive intent—namely, to describe the kind of pulses produced by portable stimulators used to reduce pain. The unit is usually connected to the skin using two or more electrodes which are typically conductive gel pads. A typical battery-operated TENS unit is able to modulate pulse width, frequency, and intensity. Generally, TENS is applied at high frequency (>50 Hz) with an intensity below motor contraction or low frequency (<10 Hz) with an intensity that produces motor contraction. More recently, many TENS units use a mixed frequency mode which alleviates tolerance to repeated use. Intensity of stimulation should be strong but comfortable with greater intensities, regardless of frequency, producing the greatest analgesia. While the use of TENS has proved effective in clinical studies, there is controversy over which conditions the device should be used to treat.
The Beck Depression Inventory, created by Aaron T. Beck, is a 21-question multiple-choice self-report inventory, one of the most widely used psychometric tests for measuring the severity of depression. Its development marked a shift among mental health professionals, who had until then, viewed depression from a psychodynamic perspective, instead of it being rooted in the patient's own thoughts.
A patient-reported outcome (PRO) is a health outcome directly reported by the patient who experienced it. It stands in contrast to an outcome reported by someone else, such as a physician-reported outcome, a nurse-reported outcome, and so on. PRO methods, such as questionnaires, are used in clinical trials or other clinical settings, to help better understand a treatment's efficacy or effectiveness. The use of digitized PROs, or electronic patient-reported outcomes (ePROs), is on the rise in today's health research setting.
The Rivermead Post-Concussion Symptoms Questionnaire, abbreviated RPQ, is a questionnaire that can be administered to someone who sustains a concussion or other form of traumatic brain injury to measure the severity of symptoms. The RPQ is used to determine the presence and severity of post-concussion syndrome (PCS), a set of somatic, cognitive, and emotional symptoms following traumatic brain injury that may persist anywhere from a week, to months, or even more than six months.
A depression rating scale is a psychometric instrument (tool), usually a questionnaire whose wording has been validated with experimental evidence, having descriptive words and phrases that indicate the severity of depression for a time period. When used, an observer may make judgements and rate a person at a specified scale level with respect to identified characteristics. Rather than being used to diagnose depression, a depression rating scale may be used to assign a score to a person's behaviour where that score may be used to determine whether that person should be evaluated more thoroughly for a depressive disorder diagnosis. Several rating scales are used for this purpose.

In general, quality of life is the perceived quality of an individual's daily life, that is, an assessment of their well-being or lack thereof. This includes all emotional, social and physical aspects of the individual's life. In health care, health-related quality of life (HRQoL) is an assessment of how the individual's well-being may be affected over time by a disease, disability or disorder.
The Patient Health Questionnaire (PHQ) is a multiple-choice self-report inventory that is used as a screening and diagnostic tool for mental health disorders of depression, anxiety, alcohol, eating, and somatoform disorders. It is the self-report version of the Primary Care Evaluation of Mental Disorders (PRIME-MD), a diagnostic tool developed in the mid-1990s by Pfizer Inc. The length of the original assessment limited its feasibility; consequently, a shorter version, consisting of 11 multi-part questions - the Patient Health Questionnaire was developed and validated.
The Ankylosing Spondylitis Quality of Life (ASQoL) questionnaire is a patient-reported outcome (PRO) measure which assesses the quality of life of patients with ankylosing spondylitis. The ASQoL is based on the needs-based quality of life model. It is a self-administered questionnaire which contains 18 items and takes up to four minutes to complete.
The Quality of Life Assessment of Growth Hormone Deficiency in Adults (QoL-AGHDA) is a disease specific patient-reported outcome measure which measures the effect growth hormone deficiency has on adult patients. The score of the QoL-AGHDA is used to determine the extent to which growth hormone deficiency has affected the patient’s quality of life, and what treatment can then be administered. A high score on the QoL-AGHDA indicates that the patient suffers from many symptoms and therefore has a lower quality of life.
The Rheumatoid Arthritis Quality of Life Questionnaire (RAQoL) is a disease-specific patient-reported outcome measure which determines the effect rheumatoid arthritis has on a patient's quality of life. The RAQoL has 30 items with a yes and no response format and takes about six minutes to complete.
The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) is a disease specific patient-reported outcome measure which assesses quality of life of patients with pulmonary hypertension (PH). It was the first pulmonary hypertension specific questionnaire for assessing patient reported symptoms, quality of life and functioning.
The Patient Reported Outcome Indices for Multiple Sclerosis (PRIMUS) is a disease specific patient-reported outcome questionnaire which measures the quality of life (QoL) of patients with multiple sclerosis.
The Asthma Life Impact Scale (ALIS) measure is a disease specific patient reported outcome questionnaire which assesses the impact that asthma has on a patient’s quality of life.
The Quality of Life In Depression Scale (QLDS), originally proposed by Sonja Hunt and Stephen McKenna, is a disease specific patient-reported outcome which assesses the impact that depression has on a patient's quality of life. It is the most commonly used measure of quality of life in clinical trials and studies of depression. The QLDS was developed as a measure to be used in future clinical trials of anti-depressant therapy.
The Quality of Life Index for Atopic Dermatitis (QoLIAD) is a disease specific patient reported outcome which measures the impact that atopic dermatitis (AD) has on a given patient's quality of life.
The Recurrent Genital Herpes Quality of Life (RGHQoL) measure is a patient-reported outcome measure which determines the impact that recurrent genital herpes has on a patient’s quality of life. It is a 20 item questionnaire with items such as “Herpes makes it difficult for me to plan ahead” and “I worry that sex will trigger an outbreak.”. Lower scores on the RGHQoL indicate a higher negative impact on quality of life.
The Unidimensional Fatigue Impact Scale (U-FIS) is a disease-specific patient-reported outcome measure which measures the impact of multiple sclerosis related fatigue. It is a 22-item unidimensional scale which is based on needs-based quality of life theory.
The Quality of Well-Being Scale (QWB) is a general health quality of life questionnaire which measures overall status and well-being over the previous three days in four areas: physical activities, social activities, mobility, and symptom/problem complexes.
References
- ↑ Hunt, S M; McKenna, S P; McEwen, J; Backett, E M; Williams, J; Papp, E (1980). "A quantitative approach to perceived health status: A validation study". Journal of Epidemiology & Community Health. 34 (4): 281–6. doi:10.1136/jech.34.4.281. JSTOR 25566204. PMC 1052092 . PMID 7241028.
- ↑ Hunt, Sonja M.; McKenna, S.P.; McEwen, J.; Williams, Jan; Papp, Evelyn (1981). "The Nottingham health profile: Subjective health status and medical consultations". Social Science & Medicine. Part A: Medical Psychology & Medical Sociology. 15 (3): 221–9. doi:10.1016/0271-7123(81)90005-5. PMID 6973203.
- ↑ Ebrahim, Shah; Barer, David; Nouri, Fiona (1986). "Use of the Nottingham Health Profile with patients after a stroke". Journal of Epidemiology & Community Health. 40 (2): 166–9. doi:10.1136/jech.40.2.166. JSTOR 25566637. PMC 1052513 . PMID 3746178.
- 1 2 Garellick, Göran; Malchau, Henrik; Herberts, Peter (1998-07-01). "Specific or general health outcome measures in the evaluation of total hip replacement". The Bone & Joint Journal. 80-B (4): 600–6. doi: 10.1302/0301-620x.80b4.8345 . PMID 9699819.
- ↑ "Nottingham Health Profile" (PDF). University of Montreal. 2001. Retrieved 16 October 2013.
- ↑ Garcia, Pilar; McCarthy, Mark (1996). Measuring Health: A Step In The Development of City Health Profiles (PDF). World Health Organization. ISBN 978-87-985788-2-6.[ page needed ]
- 1 2 Hunt, SM; McEwen, J; McKenna, SP (1985). "Measuring health status: A new tool for clinicians and epidemiologists". The Journal of the Royal College of General Practitioners. 35 (273): 185–8. PMC 1960139 . PMID 3989783.
- 1 2 "Measures Database". Galen Research. Retrieved 16 October 2013.
- ↑ Alonso, J; Anto, JM; Moreno, C (1990). "Spanish version of the Nottingham Health Profile: Translation and preliminary validity". American Journal of Public Health. 80 (6): 704–8. doi:10.2105/AJPH.80.6.704. PMC 1404742 . PMID 2343954.
- ↑ Thorsen, Hanne; McKenna, Stephen P.; Gottschalck, Lise (1993). "The Danish version of the Nottingham Health Profile: Its adaptation and reliability". Scandinavian Journal of Primary Health Care. 11 (2): 124–9. doi: 10.3109/02813439308994914 . PMID 8356362.
- ↑ Bucquet, D.; Condon, S.; Ritchie, K. (1990). "The French version of the Nottingham health profile. A comparison of items weights with those of the source version". Social Science & Medicine. 30 (7): 829–35. doi:10.1016/0277-9536(90)90207-9. PMID 2315749.
- ↑ Kohlmann, Thomas; Bullinger, Monika; Kirchberger-Blumstein, Inge (1997). "Die deutsche Version des Nottingham Health Profile (NHP): Übersetzungsmethodik und psychometrische Validierung" [German version of the Nottingham Health Profile (NHP): translation and psychometric validation]. Sozial- und Präventivmedizin (in German). 42 (3): 175–85. doi:10.1007/BF01300568. PMID 9334089.
- ↑ Mainio, Arja; Hakko, Helinä; Niemelä, Asko; Koivukangas, John; Räsänen, Pirkko (2013). "Insomnia Among Brain Tumor Patients: A Population-Based Prospective Study of Tumor Patients in Northern Finland". Journal of Psychosocial Oncology. 31 (5): 507–16. doi:10.1080/07347332.2013.822048. PMID 24010529.
- ↑ Oncu, Julide; Atamaz, Funda; Durmaz, Berrin; On, Arzu (2013). "Psychometric properties of fatigue severity and fatigue impact scales in postpolio patients". International Journal of Rehabilitation Research. 36 (4): 339–45. doi:10.1097/MRR.0b013e3283646b56. PMID 23903028.
- ↑ Santos, Ariene Angelini dos; Mansano-Schlosser, Thalyta Cristina dos Santos; Ceolim, Maria Filomena; Lost Pavarini, Sofia Cristina Iost (2013). "Sono, fragilidade e cognição: Estudo multicêntrico com idosos brasileiros". Revista Brasileira de Enfermagem. 66 (3): 351–7. doi: 10.1590/S0034-71672013000300008 . PMID 23887783.
- ↑ Johansen, K. L.; Finkelstein, F. O.; Revicki, D. A.; Evans, C.; Wan, S.; Gitlin, M.; Agodoa, I. L. (2011). "Systematic review of the impact of erythropoiesis-stimulating agents on fatigue in dialysis patients". Nephrology Dialysis Transplantation. 27 (6): 2418–25. doi:10.1093/ndt/gfr697. PMID 22187314.
- ↑ Behan, Lucy-Ann; Rogers, Bairbre; Hannon, Mark J.; O'Kelly, Patrick; Tormey, William; Smith, Diarmuid; Thompson, Christopher J.; Agha, Amar (2011). "Optimizing glucocorticoid replacement therapy in severely adrenocorticotropin-deficient hypopituitary male patients". Clinical Endocrinology. 75 (4): 505–13. doi:10.1111/j.1365-2265.2011.04074.x. PMID 21521342.
- ↑ Aydemir, G; Tezer, M S; Borman, P; Bodur, H; Unal, A (2006). "Treatment of tinnitus with transcutaneous electrical nerve stimulation improves patients' quality of life". The Journal of Laryngology & Otology. 120 (6): 442–5. doi:10.1017/S0022215106000910. PMID 16556347.