In petroleum exploration and development, formation evaluation is used to determine the ability of a borehole to produce petroleum. Essentially, it is the process of "recognizing a commercial well when you drill one".

In the oil and gas industry, the term wireline usually refers to the use of multi-conductor, single conductor or slickline cable, or "wireline", as a conveyance for the acquisition of subsurface petrophysical and geophysical data and the delivery of well construction services such as pipe recovery, perforating, plug setting and well cleaning and fishing. The subsurface geophysical and petrophysical information results in the description and analysis of subsurface geology, reservoir properties and production characteristics.
Hyperpolarization is the nuclear spin polarization of a material in a magnetic field far beyond thermal equilibrium conditions determined by the Boltzmann distribution. It can be applied to gases such as 129Xe and 3He, and small molecules where the polarization levels can be enhanced by a factor of 104-105 above thermal equilibrium levels. Hyperpolarized noble gases are typically used in magnetic resonance imaging (MRI) of the lungs. Hyperpolarized small molecules are typically used for in vivo metabolic imaging. For example, a hyperpolarized metabolite can be injected into animals or patients and the metabolic conversion can be tracked in real-time. Other applications include determining the function of the neutron spin-structures by scattering polarized electrons from a very polarized target (3He), surface interaction studies, and neutron polarizing experiments.
Well logging, also known as borehole logging is the practice of making a detailed record of the geologic formations penetrated by a borehole. The log may be based either on visual inspection of samples brought to the surface or on physical measurements made by instruments lowered into the hole. Some types of geophysical well logs can be done during any phase of a well's history: drilling, completing, producing, or abandoning. Well logging is performed in boreholes drilled for the oil and gas, groundwater, mineral and geothermal exploration, as well as part of environmental and geotechnical studies.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic field. This re-orientation occurs with absorption of electromagnetic radiation in the radio frequency region from roughly 4 to 900 MHz, which depends on the isotopic nature of the nucleus and increased proportionally to the strength of the external magnetic field. Notably, the resonance frequency of each NMR-active nucleus depends on its chemical environment. As a result, NMR spectra provide information about individual functional groups present in the sample, as well as about connections between nearby nuclei in the same molecule. As the NMR spectra are unique or highly characteristic to individual compounds and functional groups, NMR spectroscopy is one of the most important methods to identify molecular structures, particularly of organic compounds.

In Fourier transform nuclear magnetic resonance spectroscopy, free induction decay (FID) is the observable NMR signal generated by non-equilibrium nuclear spin magnetization precessing about the magnetic field. This non-equilibrium magnetization can be created generally by applying a pulse of radio-frequency close to the Larmor frequency of the nuclear spins.
Carbon-13 (C13) nuclear magnetic resonance is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is analogous to proton NMR and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms. 13C NMR detects only the 13
C
isotope. The main carbon isotope, 12
C
does not produce an NMR signal. Although ca. 1 mln. times less sensitive than 1H NMR spectroscopy, 13C NMR spectroscopy is widely used for characterizing organic and organometallic compounds, primarily because 1H-decoupled 13C-NMR spectra are more simple, have a greater sensitivity to differences in the chemical structure, and, thus, are better suited for identifying molecules in complex mixtures. At the same time, such spectra lack quantitative information about the atomic ratios of different types of carbon nuclei, because nuclear Overhauser effect used in 1H-decoupled 13C-NMR spectroscopy enhances the signals from carbon atoms with a larger number of hydrogen atoms attached to them more than from carbon atoms with a smaller number of H's, and because full relaxation of 13C nuclei is usually not attained, and the nuclei with shorter relaxation times produce more intense signals.
Nuclear magnetic resonance spectroscopy of proteins is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins, and also nucleic acids, and their complexes. The field was pioneered by Richard R. Ernst and Kurt Wüthrich at the ETH, and by Ad Bax, Marius Clore, Angela Gronenborn at the NIH, and Gerhard Wagner at Harvard University, among others. Structure determination by NMR spectroscopy usually consists of several phases, each using a separate set of highly specialized techniques. The sample is prepared, measurements are made, interpretive approaches are applied, and a structure is calculated and validated.
Two-dimensional nuclear magnetic resonance spectroscopy is a set of nuclear magnetic resonance spectroscopy (NMR) methods which give data plotted in a space defined by two frequency axes rather than one. Types of 2D NMR include correlation spectroscopy (COSY), J-spectroscopy, exchange spectroscopy (EXSY), and nuclear Overhauser effect spectroscopy (NOESY). Two-dimensional NMR spectra provide more information about a molecule than one-dimensional NMR spectra and are especially useful in determining the structure of a molecule, particularly for molecules that are too complicated to work with using one-dimensional NMR.
In MRI and NMR spectroscopy, an observable nuclear spin polarization (magnetization) is created by a homogeneous magnetic field. This field makes the magnetic dipole moments of the sample precess at the resonance (Larmor) frequency of the nuclei. At thermal equilibrium, nuclear spins precess randomly about the direction of the applied field. They become abruptly phase coherent when they are hit by radiofrequency (RF) pulses at the resonant frequency, created orthogonal to the field. The RF pulses cause the population of spin-states to be perturbed from their thermal equilibrium value. The generated transverse magnetization can then induce a signal in an RF coil that can be detected and amplified by an RF receiver. The return of the longitudinal component of the magnetization to its equilibrium value is termed spin-latticerelaxation while the loss of phase-coherence of the spins is termed spin-spin relaxation, which is manifest as an observed free induction decay (FID).
Petrophysics is the study of physical and chemical rock properties and their interactions with fluids.
Effective porosity is most commonly considered to represent the porosity of a rock or sediment available to contribute to fluid flow through the rock or sediment, or often in terms of "flow to a borehole". Porosity that is not considered "effective porosity" includes water bound to clay particles and isolated "vuggy" porosity. The effective porosity is of great importance in considering the suitability of rocks or sediments as oil or gas reservoirs, or as aquifers.
In nuclear chemistry and nuclear physics, J-couplings are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that arises from hyperfine interactions between the nuclei and local electrons. In NMR spectroscopy, J-coupling contains information about relative bond distances and angles. Most importantly, J-coupling provides information on the connectivity of chemical bonds. It is responsible for the often complex splitting of resonance lines in the NMR spectra of fairly simple molecules.
Nuclear magnetic resonance (NMR) in the geomagnetic field is conventionally referred to as Earth's field NMR (EFNMR). EFNMR is a special case of low field NMR.
Magnetization transfer (MT), in NMR and MRI, refers to the transfer of nuclear spin polarization and/or spin coherence from one population of nuclei to another population of nuclei, and to techniques that make use of these phenomena. There is some ambiguity regarding the precise definition of magnetization transfer, however the general definition given above encompasses all more specific notions. NMR active nuclei, those with non-zero spin, can be energetically coupled to one another under certain conditions. The mechanisms of nuclear-spin energy-coupling have been extensively characterized and are described in the following articles: Angular momentum coupling, Magnetic dipole–dipole interaction, J-coupling, Residual dipolar coupling, Nuclear Overhauser effect, Spin–spin relaxation, and Spin saturation transfer. Alternatively, some nuclei in a chemical system are labile and exchange between non-equivalent environments. A more specific example of this case is presented in the section Chemical Exchange Magnetization transfer.
Nuclear magnetic resonance (NMR) in porous materials covers the application of using NMR as a tool to study the structure of porous media and various processes occurring in them. This technique allows the determination of characteristics such as the porosity and pore size distribution, the permeability, the water saturation, the wettability, etc.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI). The original application of NMR to condensed matter physics is nowadays mostly devoted to strongly correlated electron systems. It reveals large manybody couplings by fast broadband detection and it should not to be confused with solid state NMR, which aims at removing the effect of the same couplings by Magic Angle Spinning techniques.
Thermoporometry and cryoporometry are methods for measuring porosity and pore-size distributions. A small region of solid melts at a lower temperature than the bulk solid, as given by the Gibbs–Thomson equation. Thus, if a liquid is imbibed into a porous material, and then frozen, the melting temperature will provide information on the pore-size distribution. The detection of the melting can be done by sensing the transient heat flows during phase transitions using differential scanning calorimetry – DSC thermoporometry, measuring the quantity of mobile liquid using nuclear magnetic resonance – NMR cryoporometry (NMRC) or measuring the amplitude of neutron scattering from the imbibed crystalline or liquid phases – ND cryoporometry (NDC).
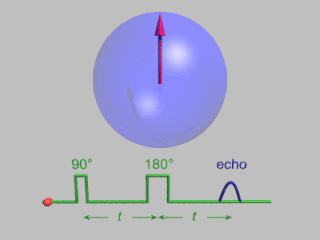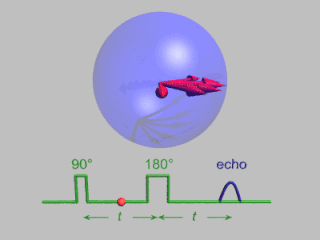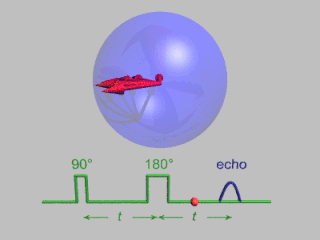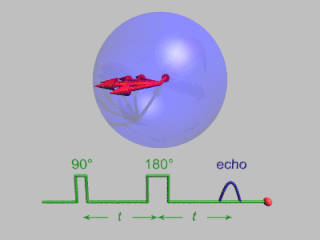
Pulsed electron paramagnetic resonance (EPR) is an electron paramagnetic resonance technique that involves the alignment of the net magnetization vector of the electron spins in a constant magnetic field. This alignment is perturbed by applying a short oscillating field, usually a microwave pulse. One can then measure the emitted microwave signal which is created by the sample magnetization. Fourier transformation of the microwave signal yields an EPR spectrum in the frequency domain. With a vast variety of pulse sequences it is possible to gain extensive knowledge on structural and dynamical properties of paramagnetic compounds. Pulsed EPR techniques such as electron spin echo envelope modulation (ESEEM) or pulsed electron nuclear double resonance (ENDOR) can reveal the interactions of the electron spin with its surrounding nuclear spins.
Surface nuclear magnetic resonance (SNMR), also known as magnetic resonance Sounding (MRS), is a geophysical technique specially designed for hydrogeology. It is based on the principle of nuclear magnetic resonance (NMR) and measurements can be used to indirectly estimate the water content of saturated and unsaturated zones in the earth's subsurface. SNMR is used to estimate aquifer properties, including the quantity of water contained in the aquifer, porosity, and hydraulic conductivity.




