An emulsion is a mixture of two or more liquids that are normally immiscible owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid is dispersed in the other. Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working.

A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more soluble in hard water, because the polar sulfonate is less likely than the polar carboxylate to bind to calcium and other ions found in hard water.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. Surfactants may function as emulsifiers, wetting agents, detergents, foaming agents, or dispersants. The word "surfactant" is a blend of surface-active agent, coined c. 1950.

An oil spill is the release of a liquid petroleum hydrocarbon into the environment, especially the marine ecosystem, due to human activity, and is a form of pollution. The term is usually given to marine oil spills, where oil is released into the ocean or coastal waters, but spills may also occur on land. Oil spills may be due to releases of crude oil from tankers, offshore platforms, drilling rigs and wells, as well as spills of refined petroleum products and their by-products, heavier fuels used by large ships such as bunker fuel, or the spill of any oily refuse or waste oil.
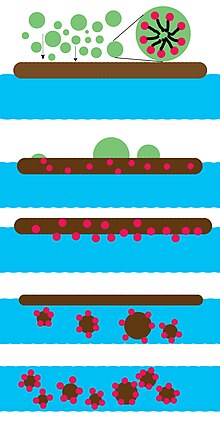
Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Rather than occurring in emulsion droplets, polymerization takes place in the latex/colloid particles that form spontaneously in the first few minutes of the process. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap'); the charge on the surfactant repels other particles electrostatically. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.
Lipophilicity, refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lipophilic, and the axiom that "like dissolves like" generally holds true. Thus lipophilic substances tend to dissolve in other lipophilic substances, but hydrophilic ("water-loving") substances tend to dissolve in water and other hydrophilic substances.

2-Butoxyethanol is an organic compound with the chemical formula BuOC2H4OH (Bu = CH3CH2CH2CH2). This colorless liquid has a sweet, ether-like odor, as it derives from the family of glycol ethers, and is a butyl ether of ethylene glycol. As a relatively nonvolatile, inexpensive solvent, it is used in many domestic and industrial products because of its properties as a surfactant. It is a known respiratory irritant and can be acutely toxic, but animal studies did not find it to be mutagenic, and no studies suggest it is a human carcinogen. A study of 13 classroom air contaminants conducted in Portugal reported a statistically significant association with increased rates of nasal obstruction and a positive association below the level of statistical significance with a higher risk of obese asthma and increased child BMI.

An amphiphile, or amphipath, is a chemical compound possessing both hydrophilic and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic compounds include surfactants. The phospholipid amphiphiles are the major structural component of cell membranes.
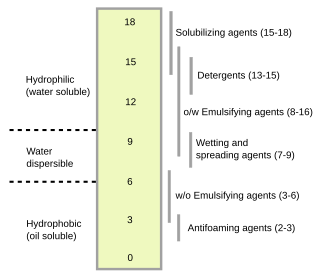
The hydrophilic–lipophilic balance (HLB) of a surfactant is a measure of its degree of hydrophilicity or lipophilicity, determined by calculating percentages of molecular weights for the hydrophilic and lipophilic portions of the surfactant molecule, as described by Griffin in 1949 and 1954. Other methods have been suggested, notably in 1957 by Davies.
A dispersant or a dispersing agent is a substance, typically a surfactant, that is added to a suspension of solid or liquid particles in a liquid to improve the separation of the particles and to prevent their settling or clumping.

The Deepwater Horizon oil spill was an industrial disaster that began on 20 April 2010 off of the coast of the United States in the Gulf of Mexico on the BP-operated Macondo Prospect, considered to be the largest marine oil spill in the history of the petroleum industry and estimated to be 8 to 31 percent larger in volume than the previous largest, the Ixtoc I oil spill, also in the Gulf of Mexico. The United States federal government estimated the total discharge at 4.9 MMbbl. After several failed efforts to contain the flow, the well was declared sealed on 19 September 2010. Reports in early 2012 indicated that the well site was still leaking. The Deepwater Horizon oil spill is regarded as one of the largest environmental disasters in world history.

Corexit is a product line of oil dispersants used during oil spill response operations. It is produced by Nalco Holding Company, an indirect subsidiary of Ecolab. Corexit was originally developed by the Standard Oil Company of New Jersey. Corexit is typically applied by aerial spraying or spraying from ships directly onto an oil slick. On contact with the dispersant, oil that would otherwise float on the surface of the water is emulsified into tiny droplets and sinks or remains suspended in the water. In theory this allows the oil to be more rapidly degraded by bacteria (bioremediation) and prevents it from accumulating on beaches and in marshes.
Dispersit SPC 1000 or Dispersit is a dispersant used for oil spills, produced by U.S. Polychemical Corporation.
Paint has four major components: pigments, binders, solvents, and additives. Pigments serve to give paint its color, texture, toughness, as well as determining if a paint is opaque or not. Common white pigments include titanium dioxide and zinc oxide. Binders are the film forming component of a paint as it dries and affects the durability, gloss, and flexibility of the coating. Polyurethanes, polyesters, and acrylics are all examples of common binders. The solvent is the medium in which all other components of the paint are dissolved and evaporates away as the paint dries and cures. The solvent also modifies the curing rate and viscosity of the paint in its liquid state. There are two types of paint: solvent-borne and water-borne paints. Solvent-borne paints use organic solvents as the primary vehicle carrying the solid components in a paint formulation, whereas water-borne paints use water as the continuous medium. The additives that are incorporated into paints are a wide range of things which impart important effects on the properties of the paint and the final coating. Common paint additives are catalysts, thickeners, stabilizers, emulsifiers, texturizers, biocides to fight bacterial growth, etc.
The Health consequences of the Deepwater Horizon oil spill are health effects related to the explosion of the Deepwater Horizon offshore drilling rig in the Gulf of Mexico on April 20, 2010. An oil discharge continued for 84 days, resulting in the largest oil spill in the history of the petroleum industry, estimated at approximately 206 million gallons. The spill exposed thousands of area residents and cleanup workers to risks associated with oil fumes, particulate matter from Controlled burns, volatile organic compounds (VOCs), polycylic aromatic hydrocarbons (PAHs), and heavy metals.

The Deepwater Horizon oil spill occurred between 10 April and 19 September 2010 in the Gulf of Mexico. A variety of techniques were used to address fundamental strategies for addressing the spilled oil, which were: to contain oil on the surface, dispersal, and removal. While most of the oil drilled off Louisiana is a lighter crude, the leaking oil was of a heavier blend which contained asphalt-like substances. According to Ed Overton, who heads a federal chemical hazard assessment team for oil spills, this type of oil emulsifies well. Once it becomes emulsified, it no longer evaporates as quickly as regular oil, does not rinse off as easily, cannot be broken down by microbes as easily, and does not burn as well. "That type of mixture essentially removes all the best oil clean-up weapons", Overton said.
On May 1, 2010, a ruptured ExxonMobil pipeline in the state of Akwa Ibom, Nigeria, spilled more than a million gallons into the delta and contributed to the major environmental issues in the Niger Delta. The spill had occurred at an Exxon platform some 20–25 miles (32–40 km) offshore which feeds the Qua Iboe oil export terminal. Exxon Mobil declared force majeure on Qua Iboe oil shipments due to the pipeline damage. The leakage in the Qua Iboe oil field discharged about 232 barrels of crude into the Atlantic Ocean contaminating the waters and coastal settlements in the predominantly fishing communities along Akwa Ibom and Cross River.
Oil pollution toxicity to marine fish has been observed from oil spills such as the Exxon Valdez disaster, and from nonpoint sources, such as surface runoff, which is the largest source of oil pollution in marine waters.

Sucrose esters or sucrose fatty acid esters are a group of non-naturally occurring surfactants chemically synthesized from the esterification of sucrose and fatty acids. This group of substances is remarkable for the wide range of hydrophilic-lipophilic balance (HLB) that it covers. The polar sucrose moiety serves as a hydrophilic end of the molecule, while the long fatty acid chain serves as a lipophilic end of the molecule. Due to this amphipathic property, sucrose esters act as emulsifiers; i.e., they have the ability to bind both water and oil simultaneously. Depending on the HLB value, some can be used as water-in-oil emulsifiers, and some as oil-in-water emulsifiers. Sucrose esters are used in cosmetics, food preservatives, food additives, and other products. A class of sucrose esters with highly substituted hydroxyl groups, olestra, is also used as a fat replacer in food.

Wetting solutions are liquids containing active chemical compounds that minimise the distance between two immiscible phases by lowering the surface tension to induce optimal spreading. The two phases, known as an interface, can be classified into five categories, namely, solid-solid, solid-liquid, solid-gas, liquid-liquid and liquid-gas.