Related Research Articles

Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil.

Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Hydrazine is an inorganic compound with the chemical formula N2H4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydrazine hydrate.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process.

Sodium hypochlorite is an alkaline inorganic chemical compound with the formula NaOCl. It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of sodium cations and hypochlorite anions.
The Solvay process or ammonia–soda process is the major industrial process for the production of sodium carbonate (soda ash, Na2CO3). The ammonia–soda process was developed into its modern form by the Belgian chemist Ernest Solvay during the 1860s. The ingredients for this are readily available and inexpensive: salt brine (from inland sources or from the sea) and limestone (from quarries). The worldwide production of soda ash in 2005 was estimated at 42 million tonnes, which is more than six kilograms (13 lb) per year for each person on Earth. Solvay-based chemical plants now produce roughly three-quarters of this supply, with the remaining being mined from natural deposits. This method superseded the Leblanc process.

In chemistry, hypochlorite, or chloroxide is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite and calcium hypochlorite. The Cl-O distance in ClO− is 1.69 Å.
The chloralkali process is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide, which are commodity chemicals required by industry. Thirty five million tons of chlorine were prepared by this process in 1987. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.
The peroxide process is a method for the industrial production of hydrazine.
Calcium hypochlorite is an inorganic compound with chemical formula Ca(ClO)2, also written as Ca(OCl)2. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite. "Pure" samples have 99.2% active chlorine. Given common industrial purity, an active chlorine content of 65-70% is typical. It is the main active ingredient of commercial products called bleaching powder, used for water treatment and as a bleaching agent.
Monochloramine, often called chloramine, is the chemical compound with the formula NH2Cl. Together with dichloramine (NHCl2) and nitrogen trichloride (NCl3), it is one of the three chloramines of ammonia. It is a colorless liquid at its melting point of −66 °C (−87 °F), but it is usually handled as a dilute aqueous solution, in which form it is sometimes used as a disinfectant. Chloramine is too unstable to have its boiling point measured.
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N−H bonds have been replaced by N−Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines. Chloramines are the most widely used members of the halamines.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.
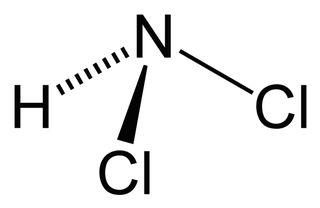
Dichloramine is a reactive inorganic compound with the chemical formula NHCl2. It is one of the three chloramines of ammonia, the others being monochloramine and nitrogen trichloride. This yellow gas is unstable and reacts with many materials. It is formed by a reaction between ammonia and chlorine or sodium hypochlorite. It is a byproduct formed during the synthesis of monochloramine and nitrogen trichloride.
In chemistry, work-up refers to the series of manipulations required to isolate and purify the product(s) of a chemical reaction. The term is used colloquially to refer to these manipulations, which may include:

Friedrich August Raschig was a German chemist and politician. He was born in Brandenburg an der Havel. After he received his PhD in 1884 from the University of Berlin for his work with Robert Wilhelm Bunsen, he started working at the BASF company. In 1891 he opened his own chemical company in Ludwigshafen am Rhein. He patented a number of chemical processes, particularly relating to phenols, one of which is now known as the Raschig phenol process, and nitrogen compounds—the Raschig process for producing hydroxylamine and the Olin Raschig process for producing hydrazine. He also developed improvements to distillation, in particular the Raschig ring, small metal or ceramic rings which are used in commercial fractional distillation columns.
Electrochlorination is the process of producing hypochlorite by passing electric current through salt water. This disinfects the water and makes it safe for human use, such as for drinking water or swimming pools.
The Raschig–Hooker process is a chemical process for the production of chlorobenzene and phenol.
Potassium hypochlorite is a chemical compound with the chemical formula KOCl, also written as KClO. It is the potassium salt of hypochlorous acid. It consists of potassium cations and hypochlorite anions. It is used in variable concentrations, often diluted in water solution. Its aqueous solutions are colorless liquids that have a strong chlorine smell. It is used as a biocide and disinfectant.

Chlorine-releasing compounds, also known as chlorine base compounds, is jargon to describe certain chlorine-containing substances that are used as disinfectants and bleaches. They include the following chemicals: sodium hypochlorite, chloramine, halazone, and sodium dichloroisocyanurate. They are widely used to disinfect water and medical equipment, and surface areas as well as bleaching materials such as cloth. The presence of organic matter can make them less effective as disinfectants. They come as a liquid solution, or as a powder that is mixed with water before use.
References
- ↑ DEpatent 192783, Friedrich Raschig,"Verfahren zur Darstellung von Hydrazin.",issued 1906-11-23
- 1 2 Schirmann, Jean-Pierre; Bourdauducq, Paul (2001). "Hydrazine". Ullmann's Encyclopedia of Industrial Chemistry . doi:10.1002/14356007.a13_177. ISBN 3527306730.
- ↑ FRpatent 382357, Friedrich Raschig,"Procédé de production de l'hydrazine",issued 1908-02-05