Related Research Articles

Microscopy is the technical field of using microscopes to view objects and areas of objects that cannot be seen with the naked eye. There are three well-known branches of microscopy: optical, electron, and scanning probe microscopy, along with the emerging field of X-ray microscopy.

A microscope is a laboratory instrument used to examine objects that are too small to be seen by the naked eye. Microscopy is the science of investigating small objects and structures using a microscope. Microscopic means being invisible to the eye unless aided by a microscope.

Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body.

The optical microscope, also referred to as a light microscope, is a type of microscope that commonly uses visible light and a system of lenses to generate magnified images of small objects. Optical microscopes are the oldest design of microscope and were possibly invented in their present compound form in the 17th century. Basic optical microscopes can be very simple, although many complex designs aim to improve resolution and sample contrast.

Single-photon emission computed tomography is a nuclear medicine tomographic imaging technique using gamma rays. It is very similar to conventional nuclear medicine planar imaging using a gamma camera, but is able to provide true 3D information. This information is typically presented as cross-sectional slices through the patient, but can be freely reformatted or manipulated as required.

Tomography is imaging by sections or sectioning that uses any kind of penetrating wave. The method is used in radiology, archaeology, biology, atmospheric science, geophysics, oceanography, plasma physics, materials science, cosmochemistry, astrophysics, quantum information, and other areas of science. The word tomography is derived from Ancient Greek τόμος tomos, "slice, section" and γράφω graphō, "to write" or, in this context as well, "to describe." A device used in tomography is called a tomograph, while the image produced is a tomogram.
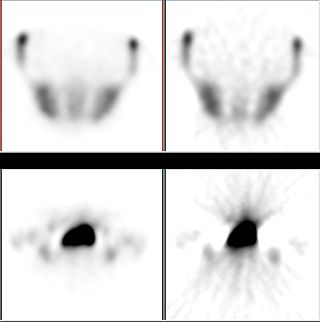
Iterative reconstruction refers to iterative algorithms used to reconstruct 2D and 3D images in certain imaging techniques. For example, in computed tomography an image must be reconstructed from projections of an object. Here, iterative reconstruction techniques are usually a better, but computationally more expensive alternative to the common filtered back projection (FBP) method, which directly calculates the image in a single reconstruction step. In recent research works, scientists have shown that extremely fast computations and massive parallelism is possible for iterative reconstruction, which makes iterative reconstruction practical for commercialization.
Medical optical imaging is the use of light as an investigational imaging technique for medical applications, pioneered by American Physical Chemist Britton Chance. Examples include optical microscopy, spectroscopy, endoscopy, scanning laser ophthalmoscopy, laser Doppler imaging, and optical coherence tomography. Because light is an electromagnetic wave, similar phenomena occur in X-rays, microwaves, and radio waves.
Computed tomography laser mammography (CTLM) is the trademark of Imaging Diagnostic Systems, Inc. for its optical tomographic technique for female breast imaging.

Molecular imaging is a field of medical imaging that focuses on imaging molecules of medical interest within living patients. This is in contrast to conventional methods for obtaining molecular information from preserved tissue samples, such as histology. Molecules of interest may be either ones produced naturally by the body, or synthetic molecules produced in a laboratory and injected into a patient by a doctor. The most common example of molecular imaging used clinically today is to inject a contrast agent into a patient's bloodstream and to use an imaging modality to track its movement in the body. Molecular imaging originated from the field of radiology from a need to better understand fundamental molecular processes inside organisms in a noninvasive manner.
Chemical imaging is the analytical capability to create a visual image of components distribution from simultaneous measurement of spectra and spatial, time information. Hyperspectral imaging measures contiguous spectral bands, as opposed to multispectral imaging which measures spaced spectral bands.
Terahertz tomography is a class of tomography where sectional imaging is done by terahertz radiation. Terahertz radiation is electromagnetic radiation with a frequency between 0.1 and 10 THz; it falls between radio waves and light waves on the spectrum; it encompasses portions of the millimeter waves and infrared wavelengths. Because of its high frequency and short wavelength, terahertz wave has a high signal-to-noise ratio in the time domain spectrum. Tomography using terahertz radiation can image samples that are opaque in the visible and near-infrared regions of the spectrum. Terahertz wave three-dimensional (3D) imaging technology has developed rapidly since its first successful application in 1997, and a series of new 3D imaging technologies have been proposed successively.
Functional imaging is a medical imaging technique of detecting or measuring changes in metabolism, blood flow, regional chemical composition, and absorption.
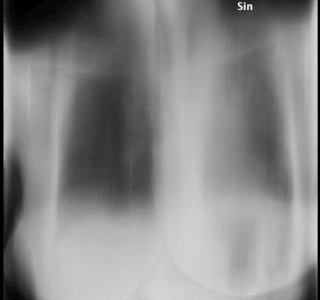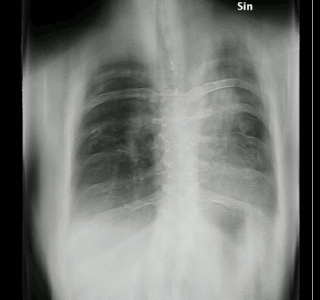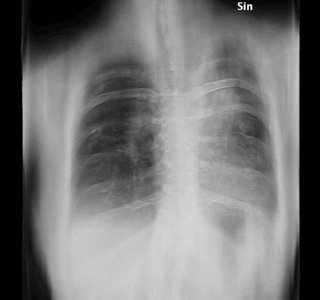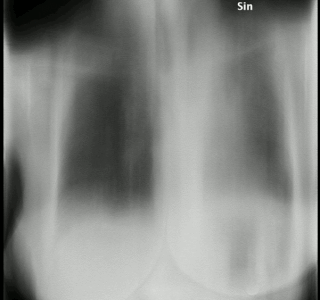
Tomosynthesis, also digital tomosynthesis (DTS), is a method for performing high-resolution limited-angle tomography at radiation dose levels comparable with projectional radiography. It has been studied for a variety of clinical applications, including vascular imaging, dental imaging, orthopedic imaging, mammographic imaging, musculoskeletal imaging, and chest imaging.

Optical sectioning is the process by which a suitably designed microscope can produce clear images of focal planes deep within a thick sample. This is used to reduce the need for thin sectioning using instruments such as the microtome. Many different techniques for optical sectioning are used and several microscopy techniques are specifically designed to improve the quality of optical sectioning.

The computed tomography imaging spectrometer (CTIS) is a snapshot imaging spectrometer which can produce in fine the three-dimensional hyperspectral datacube of a scene.
Endomicroscopy is a technique for obtaining histology-like images from inside the human body in real-time, a process known as ‘optical biopsy’. It generally refers to fluorescence confocal microscopy, although multi-photon microscopy and optical coherence tomography have also been adapted for endoscopic use. Commercially available clinical and pre-clinical endomicroscopes can achieve a resolution on the order of a micrometre, have a field-of-view of several hundred µm, and are compatible with fluorophores which are excitable using 488 nm laser light. The main clinical applications are currently in imaging of the tumour margins of the brain and gastro-intestinal tract, particularly for the diagnosis and characterisation of Barrett’s Esophagus, pancreatic cysts and colorectal lesions. A number of pre-clinical and transnational applications have been developed for endomicroscopy as it enables researchers to perform live animal imaging. Major pre-clinical applications are in gastro-intestinal tract, toumour margin detection, uterine complications, ischaemia, live imaging of cartilage and tendon and organoid imaging.
Multi-spectral optoacoustic tomography (MSOT), also known as functional photoacoustic tomography (fPAT), is an imaging technology that generates high-resolution optical images in scattering media, including biological tissues. MSOT illuminates tissue with light of transient energy, typically light pulses lasting 1-100 nanoseconds. The tissue absorbs the light pulses, and as a result undergoes thermo-elastic expansion, a phenomenon known as the optoacoustic or photoacoustic effect. This expansion gives rise to ultrasound waves (photoechoes) that are detected and formed into an image. Image formation can be done by means of hardware or computed tomography. Unlike other types of optoacoustic imaging, MSOT involves illuminating the sample with multiple wavelengths, allowing it to detect ultrasound waves emitted by different photoabsorbing molecules in the tissue, whether endogenous or exogenous. Computational techniques such as spectral unmixing deconvolute the ultrasound waves emitted by these different absorbers, allowing each emitter to be visualized separately in the target tissue. In this way, MSOT can allow visualization of hemoglobin concentration and tissue oxygenation or hypoxia. Unlike other optical imaging methods, MSOT is unaffected by photon scattering and thus can provide high-resolution optical images deep inside biological tissues.

Photoacoustic microscopy is an imaging method based on the photoacoustic effect and is a subset of photoacoustic tomography. Photoacoustic microscopy takes advantage of the local temperature rise that occurs as a result of light absorption in tissue. Using a nanosecond pulsed laser beam, tissues undergo thermoelastic expansion, resulting in the release of a wide-band acoustic wave that can be detected using a high-frequency ultrasound transducer. Since ultrasonic scattering in tissue is weaker than optical scattering, photoacoustic microscopy is capable of achieving high-resolution images at greater depths than conventional microscopy methods. Furthermore, photoacoustic microscopy is especially useful in the field of biomedical imaging due to its scalability. By adjusting the optical and acoustic foci, lateral resolution may be optimized for the desired imaging depth.
References
- ↑ Sharpe, James; Ahlgren, Ulf; Perry, Paul; Hill, Bill; Ross, Allyson; Hecksher-Sørensen, Jacob; Baldock, Richard; Davidson, Duncan (April 2002). "Optical projection tomography as a tool for 3D microscopy and gene expression studies". Science. 296 (5567): 541–5. Bibcode:2002Sci...296..541S. doi:10.1126/science.1068206. PMID 11964482. S2CID 45436939.
- ↑ Oldham, Mark; Sakhalkar, Harshad; Wang, Ying Min; Guo, Pengyi; Oliver, Tim; Bentley, Rex; Vujaskovic, Zeljko; Dewhirst, Mark (2007). "Three-dimensional imaging of whole rodent organs using optical computed and emission tomography". Journal of Biomedical Optics. 12 (1): 014009. Bibcode:2007JBO....12a4009O. doi: 10.1117/1.2709858 . PMID 17343484.
- ↑ Cheddad, A.; Svensson, C.; Sharpe, J.; Georgsson, F.; Ahlgren, U. (January 2012). "Image Processing Assisted Algorithms for Optical Projection Tomography". IEEE Transactions on Medical Imaging. 31 (1): 1–15. doi:10.1109/TMI.2011.2161590. PMID 21768046. S2CID 19452934.