Related Research Articles
Microfluidics refers to a system that manipulates a small amount of fluids ( using small channels with sizes ten to hundreds micrometres. It is a multidisciplinary field that involves molecular analysis, biodefence, molecular biology, and microelectronics. It has practical applications in the design of systems that process low volumes of fluids to achieve multiplexing, automation, and high-throughput screening. Microfluidics emerged in the beginning of the 1980s and is used in the development of inkjet printheads, DNA chips, lab-on-a-chip technology, micro-propulsion, and micro-thermal technologies.

Digital microfluidics (DMF) is a platform for lab-on-a-chip systems that is based upon the manipulation of microdroplets. Droplets are dispensed, moved, stored, mixed, reacted, or analyzed on a platform with a set of insulated electrodes. Digital microfluidics can be used together with analytical analysis procedures such as mass spectrometry, colorimetry, electrochemical, and electrochemiluminescense.

Electrophoresis is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. Electrophoresis of positively charged particles (cations) is sometimes called cataphoresis, while electrophoresis of negatively charged particles (anions) is sometimes called anaphoresis.
Electrowetting is the modification of the wetting properties of a surface with an applied electric field.

Dielectrophoresis (DEP) is a phenomenon in which a force is exerted on a dielectric particle when it is subjected to a non-uniform electric field. This force does not require the particle to be charged. All particles exhibit dielectrophoretic activity in the presence of electric fields. However, the strength of the force depends strongly on the medium and particles' electrical properties, on the particles' shape and size, as well as on the frequency of the electric field. Consequently, fields of a particular frequency can manipulate particles with great selectivity. This has allowed, for example, the separation of cells or the orientation and manipulation of nanoparticles and nanowires. Furthermore, a study of the change in DEP force as a function of frequency can allow the electrical properties of the particle to be elucidated.
An electride is an ionic compound in which an electron is the anion. Solutions of alkali metals in ammonia are electride salts. In the case of sodium, these blue solutions consist of [Na(NH3)6]+ and solvated electrons:
Extraordinary optical transmission (EOT) is the phenomenon of greatly enhanced transmission of light through a subwavelength aperture in an otherwise opaque metallic film which has been patterned with a regularly repeating periodic structure. Generally when light of a certain wavelength falls on a subwavelength aperture, it is diffracted isotropically in all directions evenly, with minimal far-field transmission. This is the understanding from classical aperture theory as described by Bethe. In EOT however, the regularly repeating structure enables much higher transmission efficiency to occur, up to several orders of magnitude greater than that predicted by classical aperture theory. It was first described in 1998.

Germanium telluride (GeTe) is a chemical compound of germanium and tellurium and is a component of chalcogenide glasses. It shows semimetallic conduction and ferroelectric behaviour.

Discrete dipole approximation (DDA), also known as coupled dipole approximation, is a method for computing scattering of radiation by particles of arbitrary shape and by periodic structures. Given a target of arbitrary geometry, one seeks to calculate its scattering and absorption properties by an approximation of the continuum target by a finite array of small polarizable dipoles. This technique is used in a variety of applications including nanophotonics, radar scattering, aerosol physics and astrophysics.

A transition-edge sensor (TES) is a type of cryogenic energy sensor or cryogenic particle detector that exploits the strongly temperature-dependent resistance of the superconducting phase transition.

Single-walled carbon nanohorn is the name given by Sumio Iijima and colleagues in 1999 to horn-shaped sheath aggregate of graphene sheets. Very similar structures had been observed in 1994 by Peter J.F. Harris, Edman Tsang, John Claridge and Malcolm Green. Ever since the discovery of the fullerene, the family of carbon nanostructures has been steadily expanded. Included in this family are single-walled and multi-walled carbon nanotubes, carbon onions and cones and, most recently, SWNHs. These SWNHs with about 40–50 nm in tubule length and about 2–3 nm in diameter are derived from SWNTs and ended by a five-pentagon conical cap with a cone opening angle of ~20o. Moreover, thousands of SWNHs associate with each other to form the ‘dahlia-like' and ‘bud-like’ structured aggregates which have an average diameter of about 80–100 nm. The former consists of tubules and graphene sheets protruding from its surface like petals of a dahlia, while the latter is composed of tubules developing inside the particle itself. Their unique structures with high surface area and microporosity make SWNHs become a promising material for gas adsorption, biosensing, drug delivery, gas storage and catalyst support for fuel cell. Single-walled carbon nanohorns are an example of the family of carbon nanocones.

Renato Zenobi is a Swiss chemist. He is Professor of Chemistry at ETH Zurich. Throughout his career, Zenobi has contributed to the field of analytical chemistry.
Differential dynamic microscopy (DDM) is an optical technique that allows performing light scattering experiments by means of a simple optical microscope. DDM is suitable for typical soft materials such as for instance liquids or gels made of colloids, polymers and liquid crystals but also for biological materials like bacteria and cells.
Xenon monochloride (XeCl) is an exciplex which is used in excimer lasers and excimer lamps emitting near ultraviolet light at 308 nm. It is most commonly used in medicine. Xenon monochloride was first synthesized in the 1960s. Its kinetic scheme is very complex and its state changes occur on a nanosecond timescale. In the gaseous state, at least two kinds of xenon monochloride are known: XeCl and Xe
2Cl, whereas complex aggregates form in the solid state in noble gas matrices. The excited state of xenon resembles halogens and it reacts with them to form excited molecular compounds.

Tantalum(V) ethoxide is a metalorganic compound with formula Ta2(OC2H5)10, often abbreviated as Ta2(OEt)10. It is a colorless solid that dissolves in some organic solvents but hydrolyzes readily. It is used to prepare films of tantalum(V) oxide.
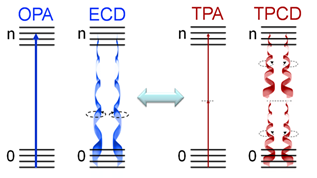
Two-photon circular dichroism (TPCD), the nonlinear counterpart of electronic circular dichroism (ECD), is defined as the differences between the two-photon absorption (TPA) cross-sections obtained using left circular polarized light and right circular polarized light.

Induced-charge electrokinetics in physics is the electrically driven fluid flow and particle motion in a liquid electrolyte. Consider a metal particle in contact with an aqueous solution in a chamber/channel. If different voltages apply to the end of this chamber/channel, electric field will generate in this chamber/channel. This applied electric field passes through this metal particle and causes the free charges inside the particle migrate under the skin of particle. As a result of this migration, the negative charges move to the side which is close to the positive voltage while the positive charges move to the opposite side of the particle. These charges under the skin of the conducting particle attract the counter-ions of the aqueous solution; thus, the electric double layer (EDL) forms around the particle. The EDL sign on the surface of the conducting particle changes from positive to negative and the distribution of the charges varies along the particle geometry. Due to these variations, the EDL is non-uniform and has different signs. Thus, the induced zeta potential around the particle, and consequently slip velocity on the surface of the particle, vary as a function of the local electric field. Differences in magnitude and direction of slip velocity on the surface of the conducting particle effects the flow pattern around this particle and causes micro vortices. Yasaman Daghighi and Dongqing Li, for the first time, experimentally illustrated these induced vortices around a 1.2mm diameter carbon-steel sphere under the 40V/cm direct current (DC) external electric filed. Chenhui Peng et al. also experimentally showed the patterns of electro-osmotic flow around an Au sphere when alternating current (AC) is involved . Electrokinetics here refers to a branch of science related to the motion and reaction of charged particles to the applied electric filed and its effects on its environment. It is sometimes referred as non-linear electrokinetic phenomena as well.
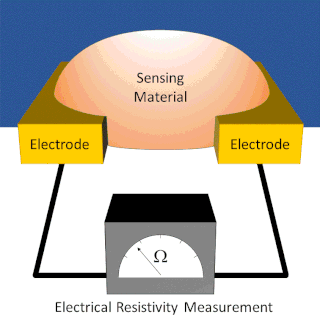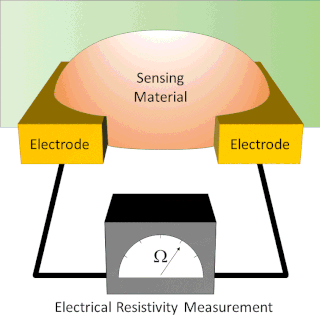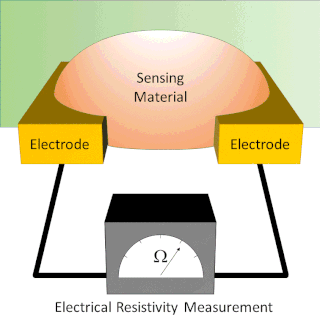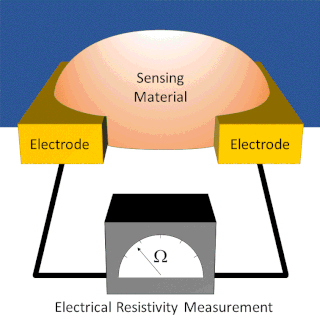
A chemiresistor is a material that changes its electrical resistance in response to changes in the nearby chemical environment. Chemiresistors are a class of chemical sensors that rely on the direct chemical interaction between the sensing material and the analyte. The sensing material and the analyte can interact by covalent bonding, hydrogen bonding, or molecular recognition. Several different materials have chemiresistor properties: metal-oxide semiconductors, some conductive polymers, and nanomaterials like graphene, carbon nanotubes and nanoparticles. Typically these materials are used as partially selective sensors in devices like electronic tongues or electronic noses.
Collective motion is defined as the spontaneous emergence of ordered movement in a system consisting of many self-propelled agents. It can be observed in everyday life, for example in flocks of birds, schools of fish, herds of animals and also in crowds and car traffic. It also appears at the microscopic level: in colonies of bacteria, motility assays and artificial self-propelled particles. The scientific community is trying to understand the universality of this phenomenon. In particular it is intensively investigated in statistical physics and in the field of active matter. Experiments on animals, biological and synthesized self-propelled particles, simulations and theories are conducted in parallel to study these phenomena. One of the most famous models that describes such behavior is the Vicsek model introduced by Tamás Vicsek et al. in 1995.
Suresh Kumar Bhatia is an Indian-born chemical engineer and professor emeritus at the School of Chemical Engineering, University of Queensland. He is known for his studies on porous media and catalytic and non-catalytic solid fluid reactions. He was awarded an ARC Australian Professorial Fellowship (2010–15) and is an elected fellow of the Indian Academy of Sciences (1993), and the Australian Academy of Technological Sciences and Engineering (2010). In 1993, the Council of Scientific and Industrial Research, the Indian government's peak agency for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, for his contributions to the engineering sciences.