Related Research Articles
In cellular biology, active transport is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate (ATP), and secondary active transport that uses an electrochemical gradient. This process is in contrast to passive transport, which allows molecules or ions to move down their concentration gradient, from an area of high concentration to an area of low concentration, without energy.

Aquaporins, also called water channels, are channel proteins from a larger family of major intrinsic proteins that form pores in the membrane of biological cells, mainly facilitating transport of water between cells. The cell membranes of a variety of different bacteria, fungi, animal and plant cells contain aquaporins through which water can flow more rapidly into and out of the cell than by diffusing through the phospholipid bilayer. Aquaporins have six membrane-spanning alpha helical domains with both carboxylic and amino terminals on the cytoplasmic side. Two hydrophobic loops contain conserved asparagine–proline–alanine which form a barrel surrounding a central pore-like region that contains additional protein density. Because aquaporins are usually always open and are prevalent in just about every cell type, this leads to a misconception that water readily passes through the cell membrane down its concentration gradient. Water can pass through the cell membrane through simple diffusion because it is a small molecule, and through osmosis, in cases where the concentration of water outside of the cell is greater than that of the inside. However, because water is a polar molecule this process of simple diffusion is relatively slow, and in tissues with high water permeability the majority of water passes through aquaporin.

An antiporter is an integral membrane protein that uses secondary active transport to move two or more molecules in opposite directions across a phospholipid membrane. It is a type of cotransporter, which means that uses the energetically favorable movement of one molecule down its electrochemical gradient to power the energetically unfavorable movement of another molecule up its electrochemical gradient. This is in contrast to symporters, which are another type of cotransporter that moves two or more ions in the same direction, and primary active transport, which is directly powered by ATP.

Cotransporters are a subcategory of membrane transport proteins (transporters) that couple the favorable movement of one molecule with its concentration gradient and unfavorable movement of another molecule against its concentration gradient. They enable coupled or cotransport and include antiporters and symporters. In general, cotransporters consist of two out of the three classes of integral membrane proteins known as transporters that move molecules and ions across biomembranes. Uniporters are also transporters but move only one type of molecule down its concentration gradient and are not classified as cotransporters.

Osmotic shock or osmotic stress is physiologic dysfunction caused by a sudden change in the solute concentration around a cell, which causes a rapid change in the movement of water across its cell membrane. Under hypertonic conditions - conditions of high concentrations of either salts, substrates or any solute in the supernatant - water is drawn out of the cells through osmosis. This also inhibits the transport of substrates and cofactors into the cell thus “shocking” the cell. Alternatively, under hypotonic conditions - when concentrations of solutes are low - water enters the cell in large amounts, causing it to swell and either burst or undergo apoptosis.
In the physiology of the kidney, tubuloglomerular feedback (TGF) is a feedback system inside the kidneys. Within each nephron, information from the renal tubules is signaled to the glomerulus. Tubuloglomerular feedback is one of several mechanisms the kidney uses to regulate glomerular filtration rate (GFR). It involves the concept of purinergic signaling, in which an increased distal tubular sodium chloride concentration causes a basolateral release of adenosine from the macula densa cells. This initiates a cascade of events that ultimately brings GFR to an appropriate level.

The Na–K–Cl cotransporter (NKCC) is a transport protein that aids in the secondary active transport of sodium, potassium, and chloride into cells. In humans there are two isoforms of this membrane transport protein, NKCC1 and NKCC2, encoded by two different genes. Two isoforms of the NKCC1/Slc12a2 gene result from keeping or skipping exon 21 in the final gene product.
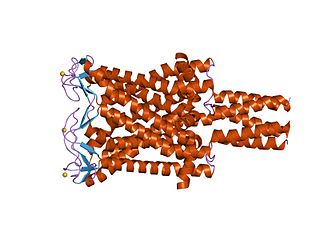
Large conductance mechanosensitive ion channels (MscLs) (TC# 1.A.22) are a family of pore-forming membrane proteins that are responsible for translating stresses at the cell membrane into an electrophysiological response. MscL has a relatively large conductance, 3 nS, making it permeable to ions, water, and small proteins when opened. MscL acts as stretch-activated osmotic release valve in response to osmotic shock.

Trimethylamine N-oxide (TMAO) is an organic compound with the formula (CH3)3NO. It is in the class of amine oxides. Although the anhydrous compound is known, trimethylamine N-oxide is usually encountered as the dihydrate. Both the anhydrous and hydrated materials are white, water-soluble solids.

Nuclear factor of activated T-cells 5, also known as NFAT5 and sometimes TonEBP, is a human gene that encodes a transcription factor that regulates the expression of genes involved in the osmotic stress.

Sodium/myo-inositol cotransporter is a protein that in humans is encoded by the SLC5A3 gene.
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration of electrolytes to keep the body fluids from becoming too diluted or concentrated. Osmotic pressure is a measure of the tendency of water to move into one solution from another by osmosis. The higher the osmotic pressure of a solution, the more water tends to move into it. Pressure must be exerted on the hypertonic side of a selectively permeable membrane to prevent diffusion of water by osmosis from the side containing pure water.
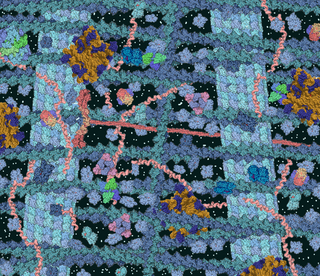
The phenomenon of macromolecular crowding alters the properties of molecules in a solution when high concentrations of macromolecules such as proteins are present. Such conditions occur routinely in living cells; for instance, the cytosol of Escherichia coli contains about 300–400 mg/ml of macromolecules. Crowding occurs since these high concentrations of macromolecules reduce the volume of solvent available for other molecules in the solution, which has the result of increasing their effective concentrations. Crowding can promote formation of a biomolecular condensate by colloidal phase separation.
Osmoprotectants or compatible solutes are small organic molecules with neutral charge and low toxicity at high concentrations that act as osmolytes and help organisms to survive in extreme osmotic stress. Osmoprotectants can be placed in three chemical classes: betaines and associated molecules, sugars and polyols, and amino acids. These molecules accumulate in cells and balance the osmotic difference between the cell's surroundings and the cytosol. In plants, their accumulation can increase survival during stresses such as drought. In extreme cases, such as in bdelloid rotifers, tardigrades, brine shrimp, and nematodes, these molecules can allow cells to survive being completely dried out and let them enter a state of suspended animation called cryptobiosis.
Mechanosensitive channels (MSCs), mechanosensitive ion channels or stretch-gated ion channels are membrane proteins capable of responding to mechanical stress over a wide dynamic range of external mechanical stimuli. They are present in the membranes of organisms from the three domains of life: bacteria, archaea, and eukarya. They are the sensors for a number of systems including the senses of touch, hearing and balance, as well as participating in cardiovascular regulation and osmotic homeostasis (e.g. thirst). The channels vary in selectivity for the permeating ions from nonselective between anions and cations in bacteria, to cation selective allowing passage Ca2+, K+ and Na+ in eukaryotes, and highly selective K+ channels in bacteria and eukaryotes.

Serine/threonine-protein kinase WNK3, also known as protein kinase lysine-deficient 3, is a protein that in humans is encoded by the WNK3 gene.
Chemical chaperones are a class of small molecules that function to enhance the folding and/or stability of proteins. Chemical chaperones are a broad and diverse group of molecules, and they can influence protein stability and polypeptide organization through a variety of mechanisms. Chemical chaperones are used for a range of applications, from production of recombinant proteins to treatment of protein misfolding in vivo.

Serine/threonine-protein kinase Sgk1 also known as serum and glucocorticoid-regulated kinase 1 is an enzyme that in humans is encoded by the SGK1 gene.
Chaperones, also called molecular chaperones, are proteins that assist other proteins in assuming their three-dimensional fold, which is necessary for protein function. However, the fold of a protein is sensitive to environmental conditions, such as temperature and pH, and thus chaperones are needed to keep proteins in their functional fold across various environmental conditions. Chaperones are an integral part of a cell's protein quality control network by assisting in protein folding and are ubiquitous across diverse biological taxa. Since protein folding, and therefore protein function, is susceptible to environmental conditions, chaperones could represent an important cellular aspect of biodiversity and environmental tolerance by organisms living in hazardous conditions. Chaperones also affect the evolution of proteins in general, as many proteins fundamentally require chaperones to fold or are naturally prone to misfolding, and therefore mitigates protein aggregation.

Sodium- and chloride-dependent betaine transporter, also known as Na(+)/Cl(-) betaine/GABA transporter (BGT-1), is a protein that in humans is encoded by the SLC6A12 gene. BGT-1 is predominantly expressed in the liver (hepatocytes). It is also expressed in the kidney where it is regulated by NFAT5 during a response to osmotic stress. Further, BGT1 is also present in the leptomeninges surrounding the brain. Deletion of the BGT1 gene in mice did not appear to have any impact on the tendency to develop epilepsy. This is to be expected considering that BGT1 is expressed at far lower levels than GAT1 and also has lower affinity for GABA. This implies that it is not likely to contribute significantly to the inactivation of the inhibitory neurotransmitter GABA.
References
- ↑ Paul H. Yancey (2005). "Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses". Journal of Experimental Biology. 208 (15): 2819–2830. doi: 10.1242/jeb.01730 . PMID 16043587.
- ↑ Review of Medical Physiology, William F. Ganong, McGraw-Hill Medical, ISBN 978-0-07-144040-0.
- ↑ Bolen DW, Baskakov IV (2001). "The osmophobic effect: natural selection of a thermodynamic force in protein folding". Journal of Molecular Biology . 310 (5): 955–963. doi:10.1006/jmbi.2001.4819. PMID 11502004.
- ↑ Su, Zhaoqian (2017). Roles of cosolvents on protein stability. OCLC 1245504372.
{{cite book}}:|work=ignored (help) - ↑ Neuhofer, W.; Beck, F. X. (2006). "Survival in Hostile Environments: Strategies of Renal Medullary Cells". Physiology. 21 (3): 171–180. doi:10.1152/physiol.00003.2006. PMID 16714475.
- 1 2 Arakawa T, Timasheff SN (1985). "The stabilization of proteins by osmolytes". Biophysical Journal . 47 (3): 411–414. Bibcode:1985BpJ....47..411A. doi:10.1016/s0006-3495(85)83932-1. PMC 1435219 . PMID 3978211.
- ↑ Csonka LN (1989). "Physiological and genetic responses of bacteria to osmotic stress". Microbiology and Molecular Biology Reviews . 53 (1): 121–147. doi:10.1128/mr.53.1.121-147.1989. PMC 372720 . PMID 2651863.
- ↑ Gallazzini, M.; Burg, M. B. (2009). "What's New About Osmotic Regulation of Glycerophosphocholine". Physiology. 24 (4): 245–249. doi:10.1152/physiol.00009.2009. PMC 2943332 . PMID 19675355.
- ↑ Yancey PH, Gerringer ME, Drazen JC, Rowden AA, Jamieson A (2014). "Marine fish may be biochemically constrained from inhabiting the deepest ocean depths". PNAS. 111 (12): 4461–4465. Bibcode:2014PNAS..111.4461Y. doi: 10.1073/pnas.1322003111 . PMC 3970477 . PMID 24591588.
- ↑ Lu, Donna (3 April 2023). "Scientists find deepest fish ever recorded at 8,300 metres underwater near Japan". The Guardian. London. Retrieved 25 May 2023.