MeNZB was a vaccine against a specific strain of group B meningococcus, used to control an epidemic of meningococcal disease in New Zealand. Most people are able to carry the meningococcus bacteria safely with no ill effects. However, meningococcal disease can cause meningitis and sepsis, resulting in brain damage, failure of various organs, severe skin and soft-tissue damage, and death.

Meningococcal disease describes infections caused by the bacterium Neisseria meningitidis. It has a high mortality rate if untreated but is vaccine-preventable. While best known as a cause of meningitis, it can also result in sepsis, which is an even more damaging and dangerous condition. Meningitis and meningococcemia are major causes of illness, death, and disability in both developed and under-developed countries.
The Joint Committee on Vaccination and Immunisation (JCVI) is an independent expert advisory committee that advises United Kingdom health departments on immunisation, making recommendations concerning vaccination schedules and vaccine safety. It has a statutory role in England and Wales, and health departments in Scotland and Northern Ireland may choose to accept its advice.

The Haemophilus influenzae type B vaccine, also known as Hib vaccine, is a vaccine used to prevent Haemophilus influenzae type b (Hib) infection. In countries that include it as a routine vaccine, rates of severe Hib infections have decreased more than 90%. It has therefore resulted in a decrease in the rate of meningitis, pneumonia, and epiglottitis.

JN-International Medical Corporation (JNIMC) is a U.S.-based biopharmaceutical corporation which since 1998 has been focused on developing vaccines and diagnostics for infectious disease for developing countries. This private corporation was founded in 1998 by Dr. Jeeri R. Reddy with the help of Dr. Kelly F. Lechtenberg in a small rural town, Oakland, Nebraska. From there it grew and expanded until in the year 2000 the corporation moved to Omaha, Nebraska.
NmVac4-A/C/Y/W-135 is the commercial name of the polysaccharide vaccine against the bacterium that causes meningococcal meningitis. The product, by JN-International Medical Corporation, is designed and formulated to be used in developing countries for protecting populations during meningitis disease epidemics.

Jeeri Reddy an American biologist who became an entrepreneur, developing new generation preventive and therapeutic vaccines. He has been an active leader in the field of the biopharmaceutical industry, commercializing diagnostics and vaccines through JN-International Medical Corporation. He is the scientific director and president of the corporation that created the world's first serological rapid tests for Tuberculosis to facilitate acid-fast bacilli microscopy for the identification of smear-positive and negative cases. Prevention of mother-to-child transmission of HIV was achieved in South East Asia by the use of rapid tests developed by Reddy in 1999. Reddy through his Corporation donated $173,050 worth of Rapid Diagnostic Tests (RDTs) for malaria in Zambia and actively participated in the prevention of child deaths due to Malaria infections. Reddy was personally invited by the president, George W. Bush, and First Lady Laura Bush to the White House for Malaria Awareness Day sponsored by US President Malaria Initiative (PMI) on Wednesday, April 25, 2007.

Meningitis is acute or chronic inflammation of the protective membranes covering the brain and spinal cord, collectively called the meninges. The most common symptoms are fever, intense headache, vomiting and neck stiffness and occasionally photophobia.
Meningococcal vaccine refers to any vaccine used to prevent infection by Neisseria meningitidis. Different versions are effective against some or all of the following types of meningococcus: A, B, C, W-135, and Y. The vaccines are between 85 and 100% effective for at least two years. They result in a decrease in meningitis and sepsis among populations where they are widely used. They are given either by injection into a muscle or just under the skin.
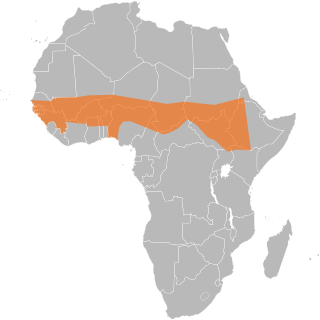
The African meningitis belt is a region in sub-Saharan Africa where the rate of incidence of meningitis is very high. It extends from Senegal to Ethiopia, and the primary cause of meningitis in the belt is Neisseria meningitidis.
The Australian Vaccination-risks Network Inc., formerly known as the Australian Vaccination-Skeptics Network (AVsN), and before that known as the Australian Vaccination Network (AVN), is an Australian anti-vaccination pressure group registered in New South Wales. As Australia's most controversial anti-vaccination organisation, it has lobbied against a variety of vaccination-related programs, downplayed the danger of childhood diseases such as measles and pertussis, championed the cause of alleged vaccination victims, and promoted the use of ineffective alternatives such as homeopathy.
MenAfriVac is a vaccine developed for use in sub-Saharan Africa for children and adults between 9 months and 29 years of age against meningococcal bacterium Neisseria meningitidis group A. The vaccine costs less than US$0.50 per dose.
Warnings About Vaccination Expectations NZ (WAVESnz), formerly the Immunisation Awareness Society (IAS), is a New Zealand anti-vaccination lobby group.
Keith Neal FFPHM FRCP DM is emeritus professor in the epidemiology of infectious diseases at the University of Nottingham. He previously worked as a consultant epidemiologist for the Field Epidemiology Service of Public Health England.
Nicola Mary Turner is a New Zealand public health advocate who is a Professor at the University of Auckland and Medical Director of the Immunisation Advisory Centre, an organisation that advises the New Zealand medical profession and the New Zealand Government. She has contributed to advisory committees for the New Zealand Ministry of Health, is a spokesperson for the Child Poverty Action Group and works in general practice. Much of her research and outreach has focused on improving immunisation coverage and closing equity gaps for the national schedule vaccine delivery in New Zealand and she has commented publicly on these issues during COVID-19 in New Zealand.
Sir Andrew John Pollard is the Ashall Professor of Infection & Immunity at the University of Oxford and a Fellow of St Cross College, Oxford. He is an Honorary Consultant Paediatrician at John Radcliffe Hospital and the Director of the Oxford Vaccine Group. He is the Chief Investigator on the University of Oxford COVID-19 Vaccine trials and has led research on vaccines for many life-threatening infectious diseases including typhoid fever, Neisseria meningitidis, Haemophilus influenzae type b, streptococcus pneumoniae, pertussis, influenza, rabies, and Ebola.
Helen Aspasia Petousis-Harris is a New Zealand vaccinologist and associate professor in the Department of General Practice and Primary Health Care at the University of Auckland. She has been involved in research related to vaccination in New Zealand since 1998, with her main areas of focus being vaccine safety and effectiveness. Petousis-Harris has had a variety of lead roles in New Zealand and international organisations that focus on vaccination and is a regular media spokesperson in this field, especially during the COVID-19 pandemic.

Shabir Ahmed Madhi, is a South African physician who is professor of vaccinology and director of the South African Medical Research Council Respiratory and Meningeal Pathogens Research Unit at the University of the Witwatersrand, and National Research Foundation/Department of Science and Technology Research Chair in Vaccine Preventable Diseases. In January 2021, he was appointed Dean of the Faculty of Health Sciences at the University of the Witwatersrand.

Immunisation against infectious disease, popularly known as The Green Book, provides information on vaccines for vaccine-preventable diseases. It acts as a guide to the UK's vaccination schedule for health professionals and health departments that give vaccines in the United Kingdom.
Helen Siobhan Marshall is an Australian medical researcher who is Professor of Vaccinology at the University of Adelaide. She was named the South Australian of the Year for 2022.









