
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R−O−R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in nonpolar organic solvents such as hexane, benzene and chloroform. Natural waxes of different types are produced by plants and animals and occur in petroleum.
Rancidification is the process of complete or incomplete autoxidation or hydrolysis of fats and oils when exposed to air, light, moisture, or bacterial action, producing short-chain aldehydes, ketones and free fatty acids.

In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.

A drying oil is an oil that hardens to a tough, solid film after a period of exposure to air, at room temperature. The oil hardens through a chemical reaction in which the components crosslink by the action of oxygen. Drying oils are a key component of oil paint and some varnishes. Some commonly used drying oils include linseed oil, tung oil, poppy seed oil, perilla oil, and walnut oil. Their use has declined over the past several decades, as they have been replaced by alkyd resins and other binders.
Biodiesel production is the process of producing the biofuel, biodiesel, through the chemical reactions of transesterification and esterification. This involves vegetable or animal fats and oils being reacted with short-chain alcohols. The alcohols used should be of low molecular weight. Ethanol is the most used because of its low cost, however, greater conversions into biodiesel can be reached using methanol. Although the transesterification reaction can be catalyzed by either acids or bases, the base-catalyzed reaction is more common. This path has lower reaction times and catalyst cost than those acid catalysis. However, alkaline catalysis has the disadvantage of high sensitivity to both water and free fatty acids present in the oils.August 10th is international biodiesel day

Saponification value or saponification number represents the number of milligrams of potassium hydroxide (KOH) or sodium hydroxide (NaOH) required to saponify one gram of fat under the conditions specified. It is a measure of the average molecular weight of all the fatty acids present in the sample in form of triglycerides. The higher the saponification value, the lower the fatty acids average length, the lighter the mean molecular weight of triglycerides and vice versa. Practically, fats or oils with high saponification value are more suitable for soap making.

Lipid peroxidation is the chain of reactions of oxidative degradation of lipids. It is the process in which free radicals "steal" electrons from the lipids in cell membranes, resulting in cell damage. This process proceeds by a free radical chain reaction mechanism. It most often affects polyunsaturated fatty acids, because they contain multiple double bonds in between which lie methylene bridges (-CH2-) that possess especially reactive hydrogen atoms. As with any radical reaction, the reaction consists of three major steps: initiation, propagation, and termination. The chemical products of this oxidation are known as lipid peroxides or lipid oxidation products (LOPs).
The Fischer–Tropsch process is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of 150–300 °C (302–572 °F) and pressures of one to several tens of atmospheres. The Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons.

In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group. If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily breaks, producing free radicals of the form RO•. Thus, organic peroxides are useful as initiators for some types of polymerization, such as the acrylic, unsaturated polyester, and vinyl ester resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can explosively combust. Organic peroxides, like their inorganic counterparts, are often powerful bleaching agents.

Linear alpha olefins (LAO) or normal alpha olefins (NAO) are olefins or alkenes with a chemical formula CxH2x, distinguished from other mono-olefins with a similar molecular formula by linearity of the hydrocarbon chain and the position of the double bond at the primary or alpha position.
Autoxidation refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organic compounds in air at ambient temperatures. Many common phenomena can be attributed to autoxidation, such as food going rancid, the 'drying' of varnishes and paints, and the perishing of rubber. It is also an important concept in both industrial chemistry and biology. Autoxidation is therefore a fairly broad term and can encompass examples of photooxygenation and catalytic oxidation.
Dibutyl ether is a chemical compound belonging to the ether family with the molecular formula of C
8H
18O. It is colorless, volatile, and flammable liquid and has peculiar ethereal smell.

Hydroperoxides or peroxols are compounds of the form ROOH, which contain the hydroperoxy functional group (–OOH). The hydroperoxide anion and the neutral hydroperoxyl radical (HOO·) consist of an unbond hydroperoxy group. When R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds.
Polymer stabilizers are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.
Catalytic oxidation are processes that rely on catalysts to introduce oxygen into organic and inorganic compounds. Many applications, including the focus of this article, involve oxidation by oxygen. Such processes are conducted on a large scale for the remediation of pollutants, production of valuable chemicals, and the production of energy.
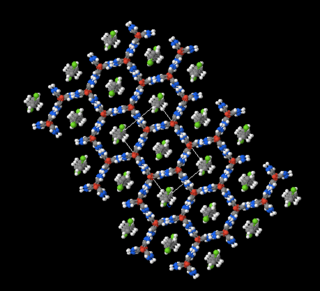
The urea extraction crystallization is a process for separating linear paraffins from hydrocarbon mixtures through the formation of urea-n-paraffin-clathrates. The process is primarily used to lower the pour point of petroleum products, by-products of the process are n-paraffins in high purity. The method may also applied for the separation of fatty acids and fatty alcohols. In addition to urea also thiourea is used in the process.
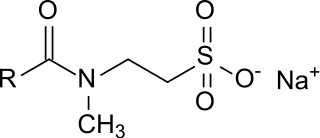
Taurates (or taurides) are a group of mild anionic surfactants. They are composed of a hydrophilic head group, consisting of N-methyltaurine (2-methylaminoethanesulfonic acid) and a lipophilic residue, consisting of a long-chain carboxylic acid (fatty acid), both linked via an amide bond. The fatty acids used could be lauric (C12), myristic (C14), palmitic (C16) or stearic acid (C18), but mainly mixtures of oleic acid (C18:1) and coconut fatty acid (C8 – C18) are used. Besides sodium, no other counterions play a relevant role (these could be e. g. ammonium or other alkali or alkaline earth metals).
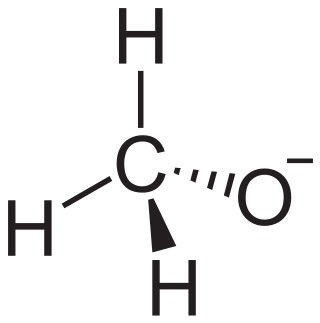
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as RO−, where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis. Transition metal alkoxides are widely used for coatings and as catalysts.















