In biology and biochemistry, protease inhibitors are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins.

Birnaviridae is a family of viruses. Salmonid fish, young sexually immature chickens, and insects serve as natural hosts. There are currently six species in this family, divided among 4 genera. Diseases associated with this family include: IPNV: infectious pancreatic necrosis in salmonid fish, causes significant losses to the aquaculture industry. chronic infection in adult, and acute viral disease in young salmonid fish.

A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta strand-turn-alpha helix structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues.
Organophosphate-induced delayed neuropathy (OPIDN), also called organophosphate-induced delayed polyneuropathy (OPIDP), is a neuropathy caused by killing of neurons in the central nervous system, especially in the spinal cord, as a result of acute or chronic organophosphate poisoning.
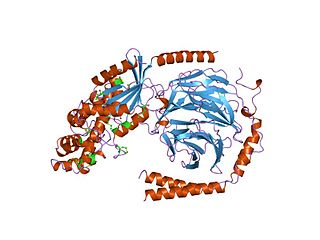
Guanine nucleotide binding proteins are membrane-associated, heterotrimeric proteins composed of three subunits: alpha, beta, and gamma. G proteins and their receptors (GPCRs) form one of the most prevalent signalling systems in mammalian cells, regulating systems as diverse as sensory perception, cell growth and hormonal regulation. At the cell surface, the binding of ligands such as hormones and neurotransmitters to a GPCR activates the receptor by causing a conformational change, which in turn activates the bound G protein on the intracellular-side of the membrane. The activated receptor promotes the exchange of bound GDP for GTP on the G protein alpha subunit. GTP binding changes the conformation of switch regions within the alpha subunit, which allows the bound trimeric G protein (inactive) to be released from the receptor, and to dissociate into active alpha subunit (GTP-bound) and beta/gamma dimer. The alpha subunit and the beta/gamma dimer go on to activate distinct downstream effectors, such as adenylyl cyclase, phosphodiesterases, phospholipase C, and ion channels. These effectors in turn regulate the intracellular concentrations of secondary messengers, such as cAMP, diacylglycerol, sodium or calcium cations, which ultimately lead to a physiological response, usually via the downstream regulation of gene transcription. The cycle is completed by the hydrolysis of alpha subunit-bound GTP to GDP, resulting in the re-association of the alpha and beta/gamma subunits and their binding to the receptor, which terminates the signal. The length of the G protein signal is controlled by the duration of the GTP-bound alpha subunit, which can be regulated by RGS proteins or by covalent modifications.
In enzymology, a lysophospholipase (EC 3.1.1.5) is an enzyme that catalyzes the chemical reaction
Secretin family receptor proteins, also known as Family B or family 2 of G-protein coupled receptors are regulated by peptide hormones from the glucagon hormone family. The family is different from adhesion G protein-coupled receptors.

Rhodopsin-like receptors are a family of proteins that comprise the largest group of G protein-coupled receptors.

Outer membrane phospholipase A1 (OMPLA) is an acyl hydrolase with a broad substrate specificity (EC:3.1.1.32.) from the bacterial outer membrane. It has been proposed that Ser164 is the active site of the protein
The class C G-protein-coupled receptors are a class of G-protein coupled receptors that include the metabotropic glutamate receptors and several additional receptors.
A neurotransmitter sodium symporter (NSS) (TC# 2.A.22) is type of neurotransmitter transporter that catalyzes the uptake of a variety of neurotransmitters, amino acids, osmolytes and related nitrogenous substances by a solute:Na+ symport mechanism. The NSS family is a member of the APC superfamily. Its constituents have been found in bacteria, archaea and eukaryotes.

Regulators of G protein signaling (RGS) are protein structural domains or the proteins that contain these domains, that function to activate the GTPase activity of heterotrimeric G-protein α-subunits.
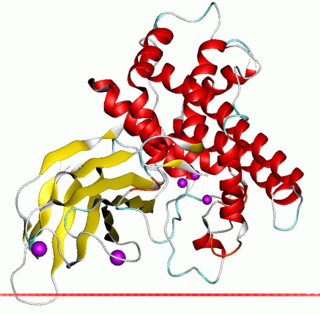
In molecular biology, zinc-dependent phospholipases C is a family of bacterial phospholipases C enzymes, some of which are also known as alpha toxins.

Adipose triglyceride lipase also known as patatin-like phospholipase domain-containing protein 2 is an enzyme that in humans is encoded by the PNPLA2 gene.

Patatin-like phospholipase domain-containing protein 3 (PNPLA3) also known as adiponutrin (ADPN), acylglycerol O-acyltransferase or calcium-independent phospholipase A2-epsilon (iPLA2-epsilon) is an enzyme that in humans is encoded by the PNPLA3 gene.

Proteins that bind cyclic nucleotides share a structural domain of about 120 residues. The best studied of these proteins is the prokaryotic catabolite gene activator where such a domain is known to be composed of three alpha-helices and a distinctive eight-stranded, antiparallel beta-barrel structure. There are six invariant amino acids in this domain, three of which are glycine residues that are thought to be essential for maintenance of the structural integrity of the beta-barrel. cAMP- and cGMP-dependent protein kinases contain two tandem copies of the cyclic nucleotide-binding domain. The cAPK's are composed of two different subunits, a catalytic chain and a regulatory chain, which contains both copies of the domain. The cGPK's are single chain enzymes that include the two copies of the domain in their N-terminal section. Vertebrate cyclic nucleotide-gated ion-channels also contain this domain. Two such cations channels have been fully characterized, one is found in rod cells where it plays a role in visual signal transduction.
The NACHT domain is an evolutionarily conserved protein domain. This NTPase domain is found in apoptosis proteins as well as those involved in MHC transcription. Its name reflects some of the proteins that contain it: NAIP, CIITA, HET-E and TEP1.

Neuropathy target esterase also known as patatin-like phospholipase domain-containing protein 6 (PNPLA6) is a neuropathy target esterase enzyme that in humans is encoded by the PNPLA6 gene.
C6orf222 is a protein that in humans is encoded by the C6orf222 gene (6p21.31). C6orf222 is conserved in mammals, birds and reptiles with the most distant ortholog being the green sea turtle,Chelonia mydas. The C6orf222 protein contains one mammalian conserved domain: DUF3293. The protein is also predicted to contain a BH3 domain, which has predicted conservation in distant orthologs from the clade Aves.














