
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture. A colloid has a dispersed phase and a continuous phase. The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre.

Centrifugation is a mechanical process which involves the use of the centrifugal force to separate particles from a solution according to their size, shape, density, medium viscosity and rotor speed. The denser components of the mixture migrate away from the axis of the centrifuge, while the less dense components of the mixture migrate towards the axis. Chemists and biologists may increase the effective gravitational force of the test tube so that the precipitate (pellet) will travel quickly and fully to the bottom of the tube. The remaining liquid that lies above the precipitate is called a supernatant or supernate.
In cell biology, cell fractionation is the process used to separate cellular components while preserving individual functions of each component. This is a method that was originally used to demonstrate the cellular location of various biochemical processes. Other uses of subcellular fractionation is to provide an enriched source of a protein for further purification, and facilitate the diagnosis of various disease states.

In biochemistry and cell biology, differential centrifugation is a common procedure used to separate organelles and other sub-cellular particles based on their sedimentation rate. Although often applied in biological analysis, differential centrifugation is a general technique also suitable for crude purification of non-living suspended particles. In a typical case where differential centrifugation is used to analyze cell-biological phenomena, a tissue sample is first lysed to break the cell membranes and release the organelles and cytosol. The lysate is then subjected to repeated centrifugations, where particles that sediment sufficiently quickly at a given centrifugal force for a given time form a compact "pellet" at the bottom of the centrifugation tube.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Ideally, to study a protein of interest, it must be separated from other components of the cell so that contaminants will not interfere in the examination of the protein of interest's structure and function. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.

Serum-separating tubes, also known as serum separator tubes or SSTs, are test tubes used in clinical chemistry tests requiring blood serum.
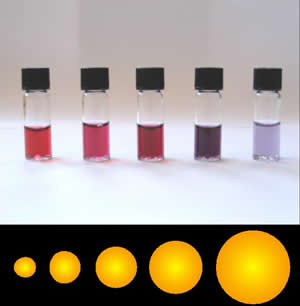
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red or blue-purple . Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine.

A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead.

A synaptosome is an isolated synaptic terminal from a neuron. Synaptosomes are obtained by mild homogenization of nervous tissue under isotonic conditions and subsequent fractionation using differential and density gradient centrifugation. Liquid shear detaches the nerve terminals from the axon and the plasma membrane surrounding the nerve terminal particle reseals. Synaptosomes are osmotically sensitive, contain numerous small clear synaptic vesicles, sometimes larger dense-core vesicles and frequently one or more small mitochondria. They carry the morphological features and most of the chemical properties of the original nerve terminal. Synaptosomes isolated from mammalian brain often retain a piece of the attached postsynaptic membrane, facing the active zone.
An isopycnic surface is a surface of constant density inside a fluid. Isopycnic surfaces contrast with isobaric or isothermal surfaces, which describe surfaces of constant pressure and constant temperature respectively. Isopycnic surfaces are sometimes referred to as "iso-density" surfaces, although this is strictly incorrect. Isopycnic typically describes surfaces, not processes. Unless there is a flux of mass into or out of a control volume, a process which occurs at a constant density also occurs at a constant volume and is called an isochoric process and not an isopycnic process.

A laboratory centrifuge is a piece of laboratory equipment, driven by a motor, which spins liquid samples at high speed. There are various types of centrifuges, depending on the size and the sample capacity.
Magnetic-activated cell sorting (MACS) is a method for separation of various cell populations depending on their surface antigens invented by Miltenyi Biotec. The name MACS is a registered trademark of the company.
Sedimentation potential occurs when dispersed particles move under the influence of either gravity or centrifugation or electricity in a medium. This motion disrupts the equilibrium symmetry of the particle's double layer. While the particle moves, the ions in the electric double layer lag behind due to the liquid flow. This causes a slight displacement between the surface charge and the electric charge of the diffuse layer. As a result, the moving particle creates a dipole moment. The sum of all of the dipoles generates an electric field which is called sedimentation potential. It can be measured with an open electrical circuit, which is also called sedimentation current.
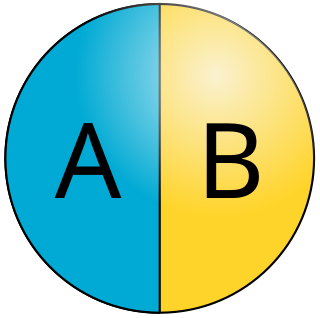
Janus particles are special types of nanoparticles or microparticles whose surfaces have two or more distinct physical properties. This unique surface of Janus particles allows two different types of chemistry to occur on the same particle. The simplest case of a Janus particle is achieved by dividing the particle into two distinct parts, each of them either made of a different material, or bearing different functional groups. For example, a Janus particle may have one half of its surface composed of hydrophilic groups and the other half hydrophobic groups, the particles might have two surfaces of different color, fluorescence, or magnetic properties. This gives these particles unique properties related to their asymmetric structure and/or functionalization.
Magnetic nanoparticles (MNPs) are a class of nanoparticle that can be manipulated using magnetic fields. Such particles commonly consist of two components, a magnetic material, often iron, nickel and cobalt, and a chemical component that has functionality. While nanoparticles are smaller than 1 micrometer in diameter, the larger microbeads are 0.5–500 micrometer in diameter. Magnetic nanoparticle clusters that are composed of a number of individual magnetic nanoparticles are known as magnetic nanobeads with a diameter of 50–200 nanometers. Magnetic nanoparticle clusters are a basis for their further magnetic assembly into magnetic nanochains. The magnetic nanoparticles have been the focus of much research recently because they possess attractive properties which could see potential use in catalysis including nanomaterial-based catalysts, biomedicine and tissue specific targeting, magnetically tunable colloidal photonic crystals, microfluidics, magnetic resonance imaging, magnetic particle imaging, data storage, environmental remediation, nanofluids, optical filters, defect sensor, magnetic cooling and cation sensors.
Sperm sorting is a means of choosing what type of sperm cell is to fertilize the egg cell. Several conventional techniques of centrifugation or swim-up. Newly applied methods such as flow cytometry expand the possibilities of sperm sorting and new techniques of sperm sorting are being developed.

A colloidal crystal is an ordered array of colloidal particles and fine grained materials analogous to a standard crystal whose repeating subunits are atoms or molecules. A natural example of this phenomenon can be found in the gem opal, where spheres of silica assume a close-packed locally periodic structure under moderate compression. Bulk properties of a colloidal crystal depend on composition, particle size, packing arrangement, and degree of regularity. Applications include photonics, materials processing, and the study of self-assembly and phase transitions.
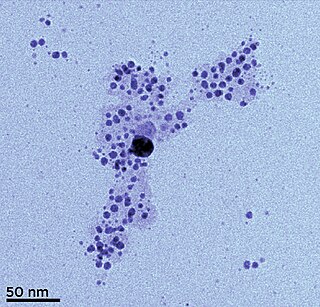
Silver nanoparticles are nanoparticles of silver of between 1 nm and 100 nm in size. While frequently described as being 'silver' some are composed of a large percentage of silver oxide due to their large ratio of surface to bulk silver atoms. Numerous shapes of nanoparticles can be constructed depending on the application at hand. Commonly used silver nanoparticles are spherical, but diamond, octagonal, and thin sheets are also common.
The Stöber process is a chemical process used to prepare silica particles of controllable and uniform size for applications in materials science. It was pioneering when it was reported by Werner Stöber and his team in 1968, and remains today the most widely used wet chemistry synthetic approach to silica nanoparticles. It is an example of a sol-gel process wherein a molecular precursor is first reacted with water in an alcoholic solution, the resulting molecules then joining together to build larger structures. The reaction produces silica particles with diameters ranging from 50 to 2000 nm, depending on conditions. The process has been actively researched since its discovery, including efforts to understand its kinetics and mechanism – a particle aggregation model was found to be a better fit for the experimental data than the initially hypothesized LaMer model. The newly acquired understanding has enabled researchers to exert a high degree of control over particle size and distribution and to fine-tune the physical properties of the resulting material in order to suit intended applications.
Nanosphere lithography (NSL) is an economical technique for generating single-layer hexagonally close packed or similar patterns of nanoscale features. Generally, NSL applies planar ordered arrays of nanometer-sized latex or silica spheres as lithography masks to fabricate nanoparticle arrays. NSL uses self-assembled monolayers of spheres as evaporation masks. These spheres can be deposited using multiple methods including Langmuir-Blodgett, dip coating, spin coating, solvent evaporation, force-assembly, and air-water interface. This method has been used to fabricate arrays of various nanopatterns, including gold nanodots with precisely controlled spacings.











