
The atomic number or proton number of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons.

A chemical element is a species of atom having the same number of protons in its atomic nuclei. For example, the atomic number of oxygen is 8, so the element oxygen describes all atoms which have 8 protons.

The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups, contain elements with similar chemical behaviours. Six groups have accepted names as well as assigned numbers: for example, group 17 elements are the halogens; and group 18 are the noble gases. Also displayed are four simple rectangular areas or blocks associated with the filling of different atomic orbitals.

Dmitri Ivanovich Mendeleev was a Russian chemist and inventor. He is best remembered for formulating the Periodic Law and creating a farsighted version of the periodic table of elements. He used the Periodic Law not only to correct the then-accepted properties of some known elements, such as the valence and atomic weight of uranium, but also to predict the properties of eight elements that were yet to be discovered.

Dmitri Mendeleev published a periodic table of the chemical elements in 1869 based on properties that appeared with some regularity as he laid out the elements from lightest to heaviest. When Mendeleev proposed his periodic table, he noted gaps in the table and predicted that then-unknown elements existed with properties appropriate to fill those gaps. He named them eka-boron, eka-aluminium and eka-silicon, with respective atomic masses of 44, 68, and 72.

In chemistry, a group is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the f-block columns are not numbered. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms, because most chemical properties are dominated by the orbital location of the outermost electron.
A period 1 element is one of the chemical elements in the first row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate periodic (recurring) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that analog elements fall into the same vertical columns. The first period contains fewer elements than any other row in the table, with only two: hydrogen and helium. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 1s orbital. Period 1 elements obey the duet rule in that they need two electrons to complete their valence shell.
A period 7 element is one of the chemical elements in the seventh row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that elements with similar behaviour fall into the same vertical columns. The seventh period contains 32 elements, tied for the most with period 6, beginning with francium and ending with oganesson, the heaviest element currently discovered. As a rule, period 7 elements fill their 7s shells first, then their 5f, 6d, and 7p shells in that order, but there are exceptions, such as uranium.

Group 3 is a group of elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group. Scandium (Sc) and yttrium (Y) are always included, but the other two spaces are usually occupied by lanthanum (La) and actinium (Ac), or by lutetium (Lu) and lawrencium (Lr); less frequently, it is considered the group should be expanded to 32 elements or contracted to contain only scandium and yttrium. When the group is understood to contain all of the lanthanides, it subsumes the rare-earth metals. Yttrium, and less frequently scandium, are sometimes also counted as rare-earth metals.

The periodic table is an arrangement of the chemical elements, which are organized on the basis of their atomic numbers, electron configurations and recurring chemical properties. Elements are presented in order of increasing atomic number. The standard form of the table consists of a grid with rows called periods and columns called groups.

Julius Lothar Meyer was a German chemist. He was one of the pioneers in developing the first periodic table of chemical elements. Both Mendeleev and Meyer worked with Robert Bunsen. He never used his first given name, and was known throughout his life simply as Lothar Meyer.

Alternative periodic tables are tabulations of chemical elements differing in their organization from the traditional depiction of the periodic system.
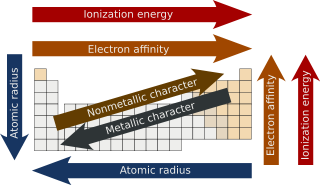
Periodic trends are specific patterns in the properties of chemical elements that are revealed in the periodic table of elements. Major periodic trends include electronegativity, ionization energy, electron affinity, atomic radii, ionic radius, metallic character, and chemical reactivity.

The abundance of elements in Earth's crust is shown in tabulated form with the estimated crustal abundance for each chemical element shown as mg/kg, or parts per million (ppm) by mass. Note that the noble gases are not included, as they form no part of the solid crust. Also not included are certain elements with extremely low crustal concentrations: technetium, promethium (61), and all elements with atomic numbers greater than 83 except thorium (90) and uranium (92).
There are currently 118 known chemical elements exhibiting many different physical and chemical properties. Amongst this diversity, scientists have found it useful to use names for various sets of elements, that illustrate similar properties, or their trends of properties. Many of these sets are formally recognized by the standards body IUPAC.

Isotopes are variants of a particular chemical element which differ in neutron number, and consequently in nucleon number. All isotopes of a given element have the same number of protons but different numbers of neutrons in each atom.

The Periodic Table: Elements with Style is a 2007 children's science book created by Simon Basher and written by Adrian Dingle. It is the first book in Basher's science series, which includes Physics: Why Matter Matters!, Biology: Life As We Know It, Astronomy: Out of this World!, Rocks and Minerals: A Gem of a Book, and Planet Earth: What Planet Are You On?, each of which is 128 pages long.
A block of the periodic table is a set of elements unified by the orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-block, p-block, d-block, and f-block.
The dividing line between metals and nonmetals can be found, in varying configurations, on some representations of the periodic table of the elements. Elements to the lower left of the line generally display increasing metallic behaviour; elements to the upper right display increasing nonmetallic behaviour. When presented as a regular stair-step, elements with the highest critical temperature for their groups lie just below the line.











