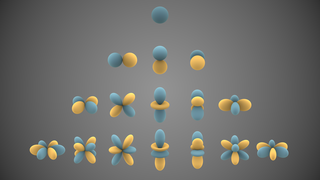The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (np) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons.
A chemical element is a chemical substance that cannot be broken down into other substances. The basic particle that constitutes a chemical element is the atom, and each chemical element is distinguished by the number of protons in the nuclei of its atoms, known as its atomic number. For example, oxygen has an atomic number of 8, meaning that each oxygen atom has 8 protons in its nucleus. This is in contrast to chemical compounds and mixtures, which contain atoms with more than one atomic number.

The noble gases make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six naturally occurring noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn).

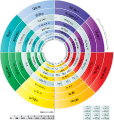
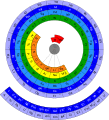
The periodic table, also known as the periodic table of the elements, arranges the chemical elements into rows ("periods") and columns ("groups"). It is an organizing icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which says that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics.

In chemistry, a transition metal is a chemical element in the d-block of the periodic table, though the elements of group 12 are sometimes excluded. The lanthanide and actinide elements are called inner transition metals and are sometimes considered to be transition metals as well.

In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively.

Dmitri Mendeleev published a periodic table of the chemical elements in 1869 based on properties that appeared with some regularity as he laid out the elements from lightest to heaviest. When Mendeleev proposed his periodic table, he noted gaps in the table and predicted that then-unknown elements existed with properties appropriate to fill those gaps. He named them eka-boron, eka-aluminium, eka-silicon, and eka-manganese, with respective atomic masses of 44, 68, 72, and 100.

In chemistry, a group is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the 14 f-block columns, between groups 2 and 3, are not numbered. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms, because most chemical properties are dominated by the orbital location of the outermost electron.

A period on the periodic table is a row of chemical elements. All elements in a row have the same number of electron shells. Each next element in a period has one more proton and is less metallic than its predecessor. Arranged this way, elements in the same group (column) have similar chemical and physical properties, reflecting the periodic law. For example, the halogens lie in the second-to-last group and share similar properties, such as high reactivity and the tendency to gain one electron to arrive at a noble-gas electronic configuration. As of 2022, a total of 118 elements have been discovered and confirmed.

A nonmetal is a chemical element that, in the broadest sense of the term, has a relatively low density and high electronegativity; they range from colorless gases to shiny solids. They are usually poor conductors of heat and electricity, and brittle or crumbly when solid due to their electrons having low mobility. In contrast, metals are good conductors and most are easily flattened into sheets and drawn into wires since their electrons are generally free-moving. Nonmetal atoms tend to attract electrons in chemical reactions and to form acidic compounds.
A period 1 element is one of the chemical elements in the first row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate periodic (recurring) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that analog elements fall into the same vertical columns. The first period contains fewer elements than any other row in the table, with only two: hydrogen and helium. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 1s orbital. Period 1 elements obey the duet rule in that they need two electrons to complete their valence shell.

The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens; although more generally the rule is applicable for the s-block and p-block of the periodic table. Other rules exist for other elements, such as the duplet rule for hydrogen and helium, or the 18-electron rule for transition metals.

Group 3 is the first group of transition metals in the periodic table. This group is closely related to the rare-earth elements. It contains the four elements scandium (Sc), yttrium (Y), lutetium (Lu), and lawrencium (Lr). The group is also called the scandium group or scandium family after its lightest member.

In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron.

The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties. In the basic form, elements are presented in order of increasing atomic number, in the reading sequence. Then, rows and columns are created by starting new rows and inserting blank cells, so that rows (periods) and columns (groups) show elements with recurring properties. For example, all elements in group (column) 18 are noble gases that are largely—though not completely—unreactive.

The aufbau principle, also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible. An example is the configuration 1s2 2s2 2p6 3s2 3p3 for the phosphorus atom, meaning that the 1s subshell has 2 electrons, and so on.

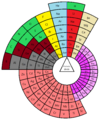
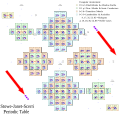
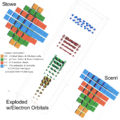
Charles Janet was a French engineer, company director, inventor and biologist. He is also known for his innovative left-step presentation of the periodic table of chemical elements.
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-block, p-block, d-block, f-block and g-block.

Eric R. Scerri is a chemist, writer and philosopher of science of Maltese origin. He is a lecturer at the University of California, Los Angeles; and the founder and editor-in-chief of Foundations of Chemistry, an international peer reviewed journal covering the history and philosophy of chemistry, and chemical education.
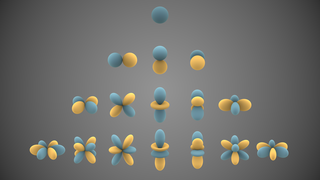
Kainosymmetry describes the first atomic orbital of each azimuthal quantum number (ℓ). Such orbitals include 1s, 2p, 3d, 4f, 5g, and so on. The term kainosymmetric was coined by Sergey Shchukarev. Pekka Pyykkö referred to such orbitals as primogenic instead. Such orbitals are much smaller than all other orbitals with the same ℓ and have no radial nodes, giving the elements that fill them special properties. They are usually less metallic than their heavier homologues, prefer lower oxidation states, and have smaller atomic and ionic radii.