| Prototype | Strukturbericht | Diagram | Lattice system | Space group | Atoms per unit cell | Coordination | notes |
|---|
| α-Pu | (none) |  | Monoclinic | P21/m
(No. 11) | 16 | | slightly distorted hexagonal structure. Lattice parameters: a = 618.3 pm, b = 482.2 pm, c = 1096.3 pm, β = 101.79° [13] [14] |
| β-S | (none) | | Monoclinic | P21/c
(No. 14) | 32 | |
| α-Np | Ac | | Orthorhombic | Pnma
(No. 62) | 8 | | highly distorted bcc structure. Lattice parameters: a = 666.3 pm, b = 472.3 pm, c = 488.7 pm [15] [16] |
| α-U | A20 |  | Orthorhombic | Cmcm
(No. 63) | 4 | Each atom has four near neighbours, 2 at 275.4 pm, 2 at 285.4 pm. The next four at distances 326.3 pm and four more at 334.2 pm. [17] | Strongly distorted hcp structure. |
| α-Ga | A11 |  | Orthorhombic | Cmce
(No. 64) | 8 | each Ga atom has one nearest neighbour at 244 pm, 2 at 270 pm, 2 at 273 pm, 2 at 279 pm. [18] | The structure is related to that of iodine. |
| b-P | A17 |  | Orthorhombic | Cmce
(No. 64) | 8 | | Specifically the black phosphorus form of phosphorus. |
| Cl | A14 |  | Orthorhombic | Cmce
(No. 64) | 8 | |
| α-S | A16 | | Orthorhombic | Fddd
(No. 70) | 16 | | |
| In | A6 |  | Tetragonal | I4/mmm
(No. 139) | 2 | | Identical symmetry to the α-Pa type structure. Can be considered slightly distorted from an ideal Cu type face-centered cubic structure [18] which has  . . |
| α-Pa | Aa | | Tetragonal | I4/mmm
(No. 139) | 2 | | Identical symmetry to the In type structure. Can be considered slightly distorted from an ideal W type body centered cubic structure which has  . . |
| β-Sn | A5 | | Tetragonal | I41/amd
(No. 141) | 4 | 4 neighbours at 302 pm; 2 at 318 pm; 4 at 377 pm; 8 at 441 pm [18] | white tin form (thermodynamical stable above 286.4 K) |
| β-B | (none) |  | Rhombohedral | R3m
(No. 166) | 105 (rh.)
315 (hex.) | | Partly due to its complexity, whether this structure is the ground state of Boron has not been fully settled. |
| α-As | A7 |  | Rhombohedral | R3m
(No. 166) | 2 (rh.)
6 (hex.) | in grey metallic form, each As atom has 3 neighbours in the same sheet at 251.7pm; 3 in adjacent sheet at 312.0 pm. [18]
each Bi atom has 3 neighbours in the same sheet at 307.2 pm; 3 in adjacent sheet at 352.9 pm. [18]
each Sb atom has 3 neighbours in the same sheet at 290.8pm; 3 in adjacent sheet at 335.5 pm. [18] | puckered sheet |
| α-Sm | (none) |  | Rhombohedral | R3m
(No. 166) | 3 (rh.)
9 (hex.) | 12 nearest neighbours | complex hcp with 9-layer repeat: ABCBCACAB.... [19] |
| α-Hg | A10 |  | Rhombohedral | R3m
(No. 166) | 1 (rh.)
3 (hex.) | 6 nearest neighbours at 234 K and 1 atm (it is liquid at room temperature and thus has no crystal structure at ambient conditions!) | Identical symmetry to the β-Po structure, distinguished based on details about the basis vectors of its unit cell. This structure can also be considered to be a distorted hcp lattice with the nearest neighbours in the same plane being approx 16% farther away [18] |
|
| β-Po | Ai | | Rhombohedral | R3m
(No. 166) | 1 (rh.)
3 (hex.) | | Identical symmetry to the α-Hg structure, distinguished based on details about the basis vectors of its unit cell. |
| γ-Se | A8 |  | Hexagonal | P321
(No. 154) | 3 | | |
| Mg | A3 | 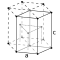 | Hexagonal | P63/mmc
(No. 194) | 2 | Zn has 6 nearest neighbors in same plane: 6 in adjacent planes 14% farther away [18]
Cd has 6 nearest neighbours in the same plane- 6 in adjacent planes 15% farther away [18] | If the unit cell axial ratio is exactly  the structure would be a mathematical hexagonal close packed (HCP) structure. However, in real materials there are deviations from this in some metals where the unit cell is distorted in one direction but the structure still retains the hcp space group—remarkable all the elements have a ratio of lattice parameters c/a < 1.633 (best are Mg and Co and worst Be with c/a ~ 1.568). In others like Zn and Cd the deviations from the ideal change the symmetry of the structure and these have a lattice parameter ratio c/a > 1.85. the structure would be a mathematical hexagonal close packed (HCP) structure. However, in real materials there are deviations from this in some metals where the unit cell is distorted in one direction but the structure still retains the hcp space group—remarkable all the elements have a ratio of lattice parameters c/a < 1.633 (best are Mg and Co and worst Be with c/a ~ 1.568). In others like Zn and Cd the deviations from the ideal change the symmetry of the structure and these have a lattice parameter ratio c/a > 1.85. |
| g-C | A9 |  | Hexagonal | P63/mmc
(No. 194) | 4 | | Specifically the graphite form of carbon. |
| α-La | A3' |  | Hexagonal | P63/mmc
(No. 194) | 4 | | The Double hexagonal close packed (DHCP) structure. Similar to the ideal hcp structure, the perfect dhcp structure should have a lattice parameter ratio of  In the real dhcp structures of 5 lanthanides (including β-Ce) In the real dhcp structures of 5 lanthanides (including β-Ce)  variates between 1.596 (Pm) and 1.6128 (Nd). For the four known actinides dhcp lattices the corresponding number vary between 1.620 (Bk) and 1.625 (Cf). [20] variates between 1.596 (Pm) and 1.6128 (Nd). For the four known actinides dhcp lattices the corresponding number vary between 1.620 (Bk) and 1.625 (Cf). [20] |
| β-N | (none) | | Hexagonal | P63/mmc
(No. 194) | 4 | | |
| α-Po | Ah |  | Cubic | Pm3m
(No. 221) | 1 | 6 nearest neighbours | simple cubic lattice. The atoms in the unit cell are at the corner of a cube. |
| γ-O | (none) |  | Cubic | Pm3n
(No. 223) | 16 | | Closely related to the β-W structure, except with a diatomic oxygen molecule in place of each tungsten atom. The molecules can rotate in place, but the direction of rotation for some of the molecules is restricted. |
| α-Mn | A12 |  | Cubic | I43m
(No. 217) | 58 | Unit cell contains Mn atoms in 4 different environments. [18] | Distorted bcc |
| W | A2 |  | Cubic | Im3m
(No. 229) | 2 | | The Body centered cubic structure (BCC). It is not a close packed structure. In this each metal atom is at the centre of a cube with 8 nearest neighbors, however the 6 atoms at the centres of the adjacent cubes are only approximately 15% further away so the coordination number can therefore be considered to be 14 when these are on one 4 fold axe structure becomes face-centred cubic (cubic close packed). |
| Cu | A1 |  | Cubic | Fm3m
(No. 225) | 4 | | The face-centered cubic (cubic close packed) structure. More content relating to number of planes within structure and implications for glide/slide e.g. ductility. |
| d-C | A4 |  | Cubic | Fd3m
(No. 227) | 8 | | The diamond cubic (DC) structure. Specifically the diamond form of Carbon. |


















