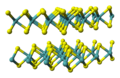A-compounds
The 'A' compounds are reserved for structures made up of atoms of all the same chemical element.
| Strukturbericht designation | Diagram | Prototype | Space group | Description |
|---|---|---|---|---|
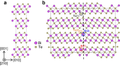
| A1 |  | Cu | Fm3m | Cubic close-packed structure (also: Face-centered cubic structure) |
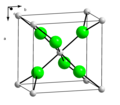
| A2 |  | W | Im3m | Body-centered cubic structure |
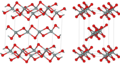
| A3 |  | Mg | P63/mmc | Hexagonal close-packed structure |
| A3' | 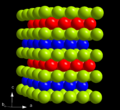 | α-La | P63/mmc | α-La structure (ABAC Barlow packing) |
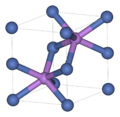
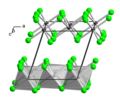
| A4 | | C (diamond) | Fd3m | Diamond cubic structure |
| A5 | β-Sn | I41/amd | ||
| A6 |  | Indium | I4/mmm | Indium structure |
| A7 |  | α-As | R3m | |
| A8 |  | gray Se | P3121 | Also called γ-Se, but that term is also used for a monoclinic form. |
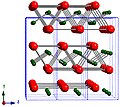
| A9 |  | C (graphite) | P63/mmc | Hexagonal graphite structure |
| A10 | α-Hg | R3m | ||
| A11 |  | α-Ga | Cmca | α-Gallium structure |
| A12 |  | α-Mn | I43m | α-Manganese structure |
| A13 |  | β-Mn | P4132 | β-Manganese structure |
| A14 |  | I2 | Cmca | Molecular iodine structure |
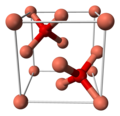
| A15 |  | β-W | Pm3n | Weaire–Phelan structure |
| A16 | α-S | Fddd | ||
| A17 |  | P | Cmca | Black phosphorus structure |
| A18 | Cl | P42/ncm | Incorrect structure [10] | |
| A19 → Ai, Ah | Po (incorrectly) | C2 | ||
| A20 |  | α-U | Cmcm | |
| Aa | α-Pa | I4/mmm | ||
| Ab | β-U | P42/mnm | σ phase | |
| Ac | α-Np | Pnma | ||
| Ad | β-Np | P4/nmm | ||
| Af | γ-HgSn6-10 | P6/mmm | ||
| Ag | B | P42/nnm | ||
| Ah |  | α-Po | Pm3m | Simple cubic structure |
| Ai | β-Po | R3m | ||
| Ak | γ-monoclinic Se | P21/c | ||
| Al | β-monoclinic Se | P21/c |