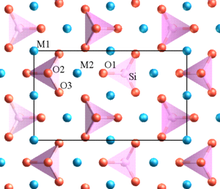
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.

The mineral olivine is a magnesium iron silicate with the chemical formula (Mg,Fe)2SiO4. It is a type of nesosilicate or orthosilicate. The primary component of the Earth's upper mantle, it is a common mineral in Earth's subsurface, but weathers quickly on the surface. For this reason, olivine has been proposed as a good candidate for accelerated weathering to sequester carbon dioxide from the Earth's oceans and atmosphere, as part of climate change mitigation. Olivine also has many other historical uses, such as the gemstone peridot, as well as industrial applications like metalworking processes.

Peridot, sometimes called chrysolite, is a yellowish-green transparent variety of olivine. Peridot is one of the few gemstones that occur in only one color.

Amphibole is a group of inosilicate minerals, forming prism or needlelike crystals, composed of double chain SiO
4 tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures. Its IMA symbol is Amp. Amphiboles can be green, black, colorless, white, yellow, blue, or brown. The International Mineralogical Association currently classifies amphiboles as a mineral supergroup, within which are two groups and several subgroups.

The pyroxenes are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula XY(Si,Al)2O6, where X represents calcium (Ca), sodium (Na), iron or magnesium (Mg) and more rarely zinc, manganese or lithium, and Y represents ions of smaller size, such as chromium (Cr), aluminium (Al), magnesium (Mg), cobalt (Co), manganese (Mn), scandium (Sc), titanium (Ti), vanadium (V) or even iron. Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica tetrahedra. Pyroxenes that crystallize in the monoclinic system are known as clinopyroxenes and those that crystallize in the orthorhombic system are known as orthopyroxenes.

Sekaninaite ((Fe+2,Mg)2Al4Si5O18) is a silicate mineral, the iron-rich analogue of cordierite.
Cement chemist notation (CCN) was developed to simplify the formulas cement chemists use on a daily basis. It is a shorthand way of writing the chemical formula of oxides of calcium, silicon, and various metals.

Enstatite is a mineral; the magnesium endmember of the pyroxene silicate mineral series enstatite (MgSiO3) – ferrosilite (FeSiO3). The magnesium rich members of the solid solution series are common rock-forming minerals found in igneous and metamorphic rocks. The intermediate composition, (Mg,Fe)SiO
3, has historically been known as hypersthene, although this name has been formally abandoned and replaced by orthopyroxene. When determined petrographically or chemically the composition is given as relative proportions of enstatite (En) and ferrosilite (Fs) (e.g., En80Fs20).

Fayalite is the iron-rich end-member of the olivine solid-solution series. In common with all minerals in the olivine group, fayalite crystallizes in the orthorhombic system with cell parameters a 4.82 Å, b 10.48 Å and c 6.09 Å.

Tephroite is the manganese endmember of the olivine group of nesosilicate minerals with the formula Mn2SiO4. A solid solution series exists between tephroite and its analogues, the group endmembers fayalite and forsterite. Divalent iron or magnesium may readily replace manganese in the olivine crystal structure.

Clinohumite is an uncommon member of the humite group, a magnesium silicate according to the chemical formula (Mg, Fe)9(SiO4)4(F,OH)2. The formula can be thought of as four olivine (Mg2SiO4), plus one brucite (Mg(OH)2). Indeed, the mineral is essentially a hydrated olivine and occurs in altered ultramafic rocks and carbonatites. Most commonly found as tiny indistinct grains, large euhedral clinohumite crystals are sought by collectors and occasionally fashioned into bright, yellow-orange gemstones. Only two sources of gem-quality material are known: the Pamir Mountains of Tajikistan, and the Taymyr region of northern Siberia. It is one of two humite group minerals that have been cut into gems, the other being the much more common chondrodite.

Komatiite is a type of ultramafic mantle-derived volcanic rock defined as having crystallised from a lava of at least 18 wt% magnesium oxide (MgO). It is classified as a 'picritic rock'. Komatiites have low silicon, potassium and aluminium, and high to extremely high magnesium content. Komatiite was named for its type locality along the Komati River in South Africa, and frequently displays spinifex texture composed of large dendritic plates of olivine and pyroxene.

Pyroxferroite (Fe2+,Ca)SiO3 is a single chain inosilicate. It is mostly composed of iron, silicon and oxygen, with smaller fractions of calcium and several other metals. Together with armalcolite and tranquillityite, it is one of the three minerals which were discovered on the Moon during the 1969 Apollo 11 mission. It was then found in Lunar and Martian meteorites as well as a mineral in the Earth's crust. Pyroxferroite can also be produced by annealing synthetic clinopyroxene at high pressures and temperatures. The mineral is metastable and gradually decomposes at ambient conditions, but this process can take billions of years.

Fractional crystallization, or crystal fractionation, is one of the most important geochemical and physical processes operating within crust and mantle of a rocky planetary body, such as the Earth. It is important in the formation of igneous rocks because it is one of the main processes of magmatic differentiation. Fractional crystallization is also important in the formation of sedimentary evaporite rocks or simply fractional crystallization is the removal of early formed crystals from an Original homogeneous magma so that the crystals are prevented from further reaction with the residual melt.
An endmember in mineralogy is a mineral that is at the extreme end of a mineral series in terms of purity of its chemical composition. Minerals often can be described as solid solutions with varying compositions of some chemical elements, rather than as substances with an exact chemical formula. There may be two or more endmembers in a group or series of minerals.

Monticellite and kirschsteinite (commonly also spelled kirschteinite) are gray silicate minerals of the olivine group with compositions CaMgSiO4 and CaFeSiO4, respectively. Most monticellites have the pure magnesium end-member composition but rare ferroan monticellites and magnesio-kirschsteinite are found with between 30 and 75 mol.% of the iron end member. Pure kirschsteinite is only found in synthetic systems. Monticellite is named after Teodoro Monticelli, an Italian mineralogist (1759–1845). Kirschsteinite is named after Egon Kirschstein, a German geologist.
Belite is an industrial mineral important in Portland cement manufacture. Its main constituent is dicalcium silicate, Ca2SiO4, sometimes formulated as 2 CaO · SiO2 (C2S in cement chemist notation).
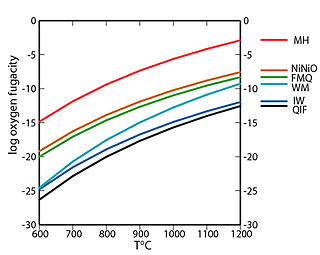
In geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.

Wadsleyite is an orthorhombic mineral with the formula β-(Mg,Fe)2SiO4. It was first found in nature in the Peace River meteorite from Alberta, Canada. It is formed by a phase transformation from olivine (α-(Mg,Fe)2SiO4) under increasing pressure and eventually transforms into spinel-structured ringwoodite (γ-(Mg,Fe)2SiO4) as pressure increases further. The structure can take up a limited amount of other bivalent cations instead of magnesium, but contrary to the α and γ structures, a β structure with the sum formula Fe2SiO4 is not thermodynamically stable. Its cell parameters are approximately a = 5.7 Å, b = 11.71 Å and c = 8.24 Å.

Ringwoodite is a high-pressure phase of Mg2SiO4 (magnesium silicate) formed at high temperatures and pressures of the Earth's mantle between 525 and 660 km (326 and 410 mi) depth. It may also contain iron and hydrogen. It is polymorphous with the olivine phase forsterite (a magnesium iron silicate).