Related Research Articles
The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Neptunium is a chemical element; it has symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. It is named after Neptune, the planet beyond Uranus in the Solar System, which uranium is named after. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. Like all actinides, it is radioactive, poisonous, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous.
In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal chain reaction can only be achieved with fissile material. The predominant neutron energy in a system may be typified by either slow neutrons or fast neutrons. Fissile material can be used to fuel thermal-neutron reactors, fast-neutron reactors and nuclear explosives.
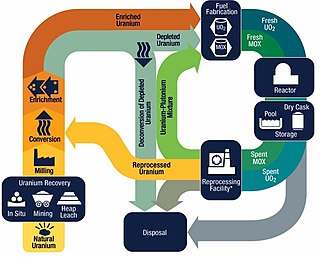
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.

Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, the reprocessed plutonium was recycled back into MOX nuclear fuel for thermal reactors. The reprocessed uranium, also known as the spent fuel material, can in principle also be re-used as fuel, but that is only economical when uranium supply is low and prices are high. Nuclear reprocessing may extend beyond fuel and include the reprocessing of other nuclear reactor material, such as Zircaloy cladding.
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the light-water reactors that predominate nuclear power generation.

A breeder reactor is a nuclear reactor that generates more fissile material than it consumes. These reactors can be fueled with more-commonly available isotopes of uranium and thorium, such as uranium-238 and thorium-232, as opposed to the rare uranium-235 which is used in conventional reactors. These materials are called fertile materials since they can be bred into fuel by these breeder reactors.

The integral fast reactor is a design for a nuclear reactor using fast neutrons and no neutron moderator. IFR would breed more fuel and is distinguished by a nuclear fuel cycle that uses reprocessing via electrorefining at the reactor site.
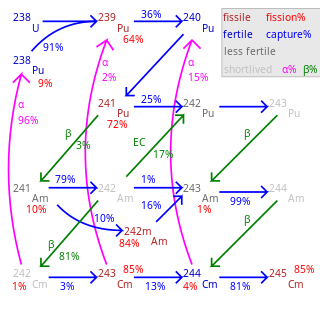
Fertile material is a material that, although not fissile itself, can be converted into a fissile material by neutron absorption.

Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in thermal spectrum nuclear reactors, along with uranium-235 and uranium-233. Plutonium-239 has a half-life of 24,110 years.
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope synthesized being plutonium-238 in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are plutonium-244 with a half-life of 80.8 million years; plutonium-242 with a half-life of 373,300 years; and plutonium-239 with a half-life of 24,110 years; and plutonium-240 with a half-life of 6,560 years. This element also has eight meta states; all have half-lives of less than one second.

The thorium fuel cycle is a nuclear fuel cycle that uses an isotope of thorium, 232
Th
, as the fertile material. In the reactor, 232
Th
is transmuted into the fissile artificial uranium isotope 233
U
which is the nuclear fuel. Unlike natural uranium, natural thorium contains only trace amounts of fissile material, which are insufficient to initiate a nuclear chain reaction. Additional fissile material or another neutron source is necessary to initiate the fuel cycle. In a thorium-fuelled reactor, 232
Th
absorbs neutrons to produce 233
U
. This parallels the process in uranium breeder reactors whereby fertile 238
U
absorbs neutrons to form fissile 239
Pu
. Depending on the design of the reactor and fuel cycle, the generated 233
U
either fissions in situ or is chemically separated from the used nuclear fuel and formed into new nuclear fuel.

Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor. It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and, depending on its point along the nuclear fuel cycle, it will have different isotopic constituents than when it started.
Weapons-grade nuclear material is any fissionable nuclear material that is pure enough to make a nuclear weapon and has properties that make it particularly suitable for nuclear weapons use. Plutonium and uranium in grades normally used in nuclear weapons are the most common examples.

Environmental radioactivity is not limited to actinides; non-actinides such as radon and radium are of note. While all actinides are radioactive, there are a lot of actinides or actinide-relating minerals in the Earth's crust such as uranium and thorium. These minerals are helpful in many ways, such as carbon-dating, most detectors, X-rays, and more.
Uranium-236 (236U) is an isotope of uranium that is neither fissile with thermal neutrons, nor very good fertile material, but is generally considered a nuisance and long-lived radioactive waste. It is found in spent nuclear fuel and in the reprocessed uranium made from spent nuclear fuel.
In nuclear power technology, burnup is a measure of how much energy is extracted from a primary nuclear fuel source. It is measured as the fraction of fuel atoms that underwent fission in %FIMA or %FIFA as well as, preferably, the actual energy released per mass of initial fuel in gigawatt-days/metric ton of heavy metal (GWd/tHM), or similar units.
Plutonium-242 is one of the isotopes of plutonium, the second longest-lived, with a half-life of 375,000 years. The half-life of 242Pu is about 15 times that of 239Pu; so it is one-fifteenth as radioactive, and not one of the larger contributors to nuclear waste radioactivity. 242Pu's gamma ray emissions are also weaker than those of the other isotopes.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
Remix Fuel was developed in Russia to make use of mixed recycled uranium and plutonium from spent nuclear fuel to manufacture fresh fuel suitable for widespread use in Russian reactor designs.