This article needs additional citations for verification .(September 2020) |
This article needs additional citations for verification .(September 2020) |
For each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy to remove a third electron from the +2 ion, and so on.
"use" and "WEL" give ionization energy in the unit kJ/mol; "CRC" gives atomic ionization energy in the unit eV. [1]
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 H hydrogen [2] [3] | ||||||||||||||||||||||||||||||
| use | 1312.0 | |||||||||||||||||||||||||||||
| WEL | 1312.0 | |||||||||||||||||||||||||||||
| CRC | 13.59844 [4] | |||||||||||||||||||||||||||||
| 2 He helium [2] [3] | ||||||||||||||||||||||||||||||
| use | 2372.3 | 5250.5 | ||||||||||||||||||||||||||||
| WEL | 2372.3 | 5250.5 | ||||||||||||||||||||||||||||
| CRC | 24.58738 [5] | 54.41776 [5] | ||||||||||||||||||||||||||||
| 3 Li lithium [2] [3] | ||||||||||||||||||||||||||||||
| use | 520.2 | 7298.1 | 11815.0 | |||||||||||||||||||||||||||
| WEL | 520.2 | 7298.1 | 11815.0 | |||||||||||||||||||||||||||
| CRC | 5.39171 [6] | 75.64009 [7] | 122.45435 [8] | |||||||||||||||||||||||||||
| 4 Be beryllium [2] [3] | ||||||||||||||||||||||||||||||
| use | 899.5 | 1757.1 | 14848.7 | 21006.6 | ||||||||||||||||||||||||||
| WEL | 899.5 | 1757.1 | 14848.7 | 21006.6 | ||||||||||||||||||||||||||
| CRC | 9.32269 [9] | 18.21115 | 153.89620 | 217.71858 | ||||||||||||||||||||||||||
| 5 B boron | ||||||||||||||||||||||||||||||
| use | 800.6 | 2427.1 | 3659.7 | 25025.8 | 32826.7 | |||||||||||||||||||||||||
| WEL | 800.6 | 2427.1 | 3659.7 | 25025.8 | 32826.7 | |||||||||||||||||||||||||
| CRC | 8.29803 | 25.15484 | 37.93064 | 259.37521 | 340.22580 | |||||||||||||||||||||||||
| 6 C carbon | ||||||||||||||||||||||||||||||
| use | 1086.5 | 2352.6 | 4620.5 | 6222.7 | 37831 | 47277.0 | ||||||||||||||||||||||||
| WEL | 1086.5 | 2352.6 | 4620.5 | 6222.7 | 37831 | 47277.0 | ||||||||||||||||||||||||
| CRC | 11.26030 | 24.38332 | 47.8878 | 64.4939 | 392.087 | 489.99334 | ||||||||||||||||||||||||
| 7 N nitrogen | ||||||||||||||||||||||||||||||
| use | 1402.3 | 2856 | 4578.1 | 7475.0 | 9444.9 | 53266.6 | 64360 | |||||||||||||||||||||||
| WEL | 1402.3 | 2856 | 4578.1 | 7475.0 | 9444.9 | 53266.6 | 64360 | |||||||||||||||||||||||
| CRC | 14.53414 | 29.6013 | 47.44924 | 77.4735 | 97.8902 | 552.0718 | 667.046 | |||||||||||||||||||||||
| 8 O oxygen | ||||||||||||||||||||||||||||||
| use | 1313.9 | 3388.3 | 5300.5 | 7469.2 | 10989.5 | 13326.5 | 71330 | 84078.0 | ||||||||||||||||||||||
| WEL | 1313.9 | 3388.3 | 5300.5 | 7469.2 | 10989.5 | 13326.5 | 71330 | 84078.0 | ||||||||||||||||||||||
| CRC | 13.61806 | 35.11730 | 54.9355 | 77.41353 | 113.8990 | 138.1197 | 739.29 | 871.4101 | ||||||||||||||||||||||
| 9 F fluorine | ||||||||||||||||||||||||||||||
| use | 1681.0 | 3374.2 | 6050.4 | 8407.7 | 11022.7 | 15164.1 | 17868 | 92038.1 | 106434.3 | |||||||||||||||||||||
| WEL | 1681.0 | 3374.2 | 6050.4 | 8407.7 | 11022.7 | 15164.1 | 17868 | 92038.1 | 106434.3 | |||||||||||||||||||||
| CRC | 17.42282 | 34.97082 | 62.7084 | 87.1398 | 114.2428 | 157.1651 | 185.186 | 953.9112 | 1103.1176 | |||||||||||||||||||||
| 10 Ne neon | ||||||||||||||||||||||||||||||
| use | 2080.7 | 3952.3 | 6122 | 9371 | 12177 | 15238 | 19999.0 | 23069.5 | 115379.5 | 131432 | ||||||||||||||||||||
| WEL | 2080.7 | 3952.3 | 6122 | 9371 | 12177 | 15238 | 19999.0 | 23069.5 | 115379.5 | 131432 | ||||||||||||||||||||
| CRC | 21.5646 | 40.96328 | 63.45 | 97.12 | 126.21 | 157.93 | 207.2759 | 239.0989 | 1195.8286 | 1362.1995 | ||||||||||||||||||||
| 11 Na sodium | ||||||||||||||||||||||||||||||
| use | 495.8 | 4562 | 6910.3 | 9543 | 13354 | 16613 | 20117 | 25496 | 28932 | 141362 | 159076 | |||||||||||||||||||
| WEL | 495.8 | 4562 | 6910.3 | 9543 | 13354 | 16613 | 20117 | 25496 | 28932 | 141362 | ||||||||||||||||||||
| CRC | 5.13908 | 47.2864 | 71.6200 | 98.91 | 138.40 | 172.18 | 208.50 | 264.25 | 299.864 | 1465.121 | 1648.702 | |||||||||||||||||||
| 12 Mg magnesium | ||||||||||||||||||||||||||||||
| use | 737.7 | 1450.7 | 7732.7 | 10542.5 | 13630 | 18020 | 21711 | 25661 | 31653 | 35458 | 169988 | 189368 | ||||||||||||||||||
| WEL | 737.7 | 1450.7 | 7732.7 | 10542.5 | 13630 | 18020 | 21711 | 25661 | 31653 | 35458 | ||||||||||||||||||||
| CRC | 7.64624 | 15.03528 | 80.1437 | 109.2655 | 141.27 | 186.76 | 225.02 | 265.96 | 328.06 | 367.50 | 1761.805 | 1962.6650 | ||||||||||||||||||
| 13 Al aluminium | ||||||||||||||||||||||||||||||
| use | 577.5 | 1816.7 | 2744.8 | 11577 | 14842 | 18379 | 23326 | 27465 | 31853 | 38473 | 42647 | 201266 | 222316 | |||||||||||||||||
| WEL | 577.5 | 1816.7 | 2744.8 | 11577 | 14842 | 18379 | 23326 | 27465 | 31853 | 38473 | ||||||||||||||||||||
| CRC | 5.98577 | 18.82856 | 28.44765 | 119.992 | 153.825 | 190.49 | 241.76 | 284.66 | 330.13 | 398.75 | 442.00 | 2085.98 | 2304.1410 | |||||||||||||||||
| 14 Si silicon | ||||||||||||||||||||||||||||||
| use | 786.5 | 1577.1 | 3231.6 | 4355.5 | 16091 | 19805 | 23780 | 29287 | 33878 | 38726 | 45962 | 50502 | 235196 | 257923 | ||||||||||||||||
| WEL | 786.5 | 1577.1 | 3231.6 | 4355.5 | 16091 | 19805 | 23780 | 29287 | 33878 | 38726 | ||||||||||||||||||||
| CRC | 8.15169 | 16.34585 | 33.49302 | 45.14181 | 166.767 | 205.27 | 246.5 | 303.54 | 351.12 | 401.37 | 476.36 | 523.42 | 2437.63 | 2673.182 | ||||||||||||||||
| 15 P phosphorus | ||||||||||||||||||||||||||||||
| use | 1011.8 | 1907 | 2914.1 | 4963.6 | 6273.9 | 21267 | 25431 | 29872 | 35905 | 40950 | 46261 | 54110 | 59024 | 271791 | 296195 | |||||||||||||||
| WEL | 1011.8 | 1907 | 2914.1 | 4963.6 | 6273.9 | 21267 | 25431 | 29872 | 35905 | 40950 | ||||||||||||||||||||
| CRC | 10.48669 | 19.7694 | 30.2027 | 51.4439 | 65.0251 | 220.421 | 263.57 | 309.60 | 372.13 | 424.4 | 479.46 | 560.8 | 611.74 | 2816.91 | 3069.842 | |||||||||||||||
| 16 S sulfur | ||||||||||||||||||||||||||||||
| use | 999.6 | 2252 | 3357 | 4556 | 7004.3 | 8495.8 | 27107 | 31719 | 36621 | 43177 | 48710 | 54460 | 62930 | 68216 | 311048 | 337138 | ||||||||||||||
| WEL | 999.6 | 2252 | 3357 | 4556 | 7004.3 | 8495.8 | 27107 | 31719 | 36621 | 43177 | ||||||||||||||||||||
| CRC | 10.36001 | 23.3379 | 34.79 | 47.222 | 72.5945 | 88.0530 | 280.948 | 328.75 | 379.55 | 447.5 | 504.8 | 564.44 | 652.2 | 707.01 | 3223.78 | 3494.1892 | ||||||||||||||
| 17 Cl chlorine | ||||||||||||||||||||||||||||||
| use | 1251.2 | 2298 | 3822 | 5158.6 | 6542 | 9362 | 11018 | 33604 | 38600 | 43961 | 51068 | 57119 | 63363 | 72341 | 78095 | 352994 | 380760 | |||||||||||||
| WEL | 1251.2 | 2298 | 3822 | 5158.6 | 6542 | 9362 | 11018 | 33604 | 38600 | 43961 | ||||||||||||||||||||
| CRC | 12.96764 | 23.814 | 39.61 | 53.4652 | 67.8 | 97.03 | 114.1958 | 348.28 | 400.06 | 455.63 | 529.28 | 591.99 | 656.71 | 749.76 | 809.40 | 3658.521 | 3946.2960 | |||||||||||||
| 18 Ar argon | ||||||||||||||||||||||||||||||
| use | 1520.6 | 2665.8 | 3931 | 5771 | 7238 | 8781 | 11995 | 13842 | 40760 | 46186 | 52002 | 59653 | 66199 | 72918 | 82473 | 88576 | 397605 | 427066 | ||||||||||||
| WEL | 1520.6 | 2665.8 | 3931 | 5771 | 7238 | 8781 | 11995 | 13842 | 40760 | 46186 | ||||||||||||||||||||
| CRC | 15.75962 | 27.62967 | 40.74 | 59.81 | 75.02 | 91.009 | 124.323 | 143.460 | 422.45 | 478.69 | 538.96 | 618.26 | 686.10 | 755.74 | 854.77 | 918.03 | 4120.8857 | 4426.2296 | ||||||||||||
| 19 K potassium | ||||||||||||||||||||||||||||||
| use | 418.8 | 3052 | 4420 | 5877 | 7975 | 9590 | 11343 | 14944 | 16963.7 | 48610 | 54490 | 60730 | 68950 | 75900 | 83080 | 93400 | 99710 | 444880 | 476063 | |||||||||||
| WEL | 418.8 | 3052 | 4420 | 5877 | 7975 | 9590 | 11343 | 14944 | 16963.7 | 48610 | ||||||||||||||||||||
| CRC | 4.34066 | 31.63 | 45.806 | 60.91 | 82.66 | 99.4 | 117.56 | 154.88 | 175.8174 | 503.8 | 564.7 | 629.4 | 714.6 | 786.6 | 861.1 | 968 | 1033.4 | 4610.8 | 4934.046 | |||||||||||
| 20 Ca calcium | ||||||||||||||||||||||||||||||
| use | 589.8 | 1145.4 | 4912.4 | 6491 | 8153 | 10496 | 12270 | 14206 | 18191 | 20385 | 57110 | 63410 | 70110 | 78890 | 86310 | 94000 | 104900 | 111711 | 494850 | 527762 | ||||||||||
| WEL | 589.8 | 1145.4 | 4912.4 | 6491 | 8153 | 10496 | 12270 | 14206 | 18191 | 20385 | 57110 | |||||||||||||||||||
| CRC | 6.11316 | 11.87172 | 50.9131 | 67.27 | 84.50 | 108.78 | 127.2 | 147.24 | 188.54 | 211.275 | 591.9 | 657.2 | 726.6 | 817.6 | 894.5 | 974 | 1087 | 1157.8 | 5128.8 | 5469.864 | ||||||||||
| 21 Sc scandium | ||||||||||||||||||||||||||||||
| use | 633.1 | 1235.0 | 2388.6 | 7090.6 | 8843 | 10679 | 13310 | 15250 | 17370 | 21726 | 24102 | 66320 | 73010 | 80160 | 89490 | 97400 | 105600 | 117000 | 124270 | 547530 | 582163 | |||||||||
| WEL | 633.1 | 1235.0 | 2388.6 | 7090.6 | 8843 | 10679 | 13310 | 15250 | 17370 | 21726 | 24102 | 66320 | 73010 | 80160 | 89490 | 97400 | 105600 | 117000 | 124270 | 547530 | 582163 | |||||||||
| CRC | 6.5615 | 12.79967 | 24.75666 | 73.4894 | 91.65 | 110.68 | 138.0 | 158.1 | 180.03 | 225.18 | 249.798 | 687.36 | 756.7 | 830.8 | 927.5 | 1009 | 1094 | 1213 | 1287.97 | 5674.8 | 6033.712 | |||||||||
| 22 Ti titanium | ||||||||||||||||||||||||||||||
| use | 658.8 | 1309.8 | 2652.5 | 4174.6 | 9581 | 11533 | 13590 | 16440 | 18530 | 20833 | 25575 | 28125 | 76015 | 83280 | 90880 | 100700 | 109100 | 117800 | 129900 | 137530 | 602930 | 639294 | ||||||||
| WEL | 658.8 | 1309.8 | 2652.5 | 4174.6 | 9581 | 11533 | 13590 | 16440 | 18530 | 20833 | 25575 | 28125 | 76015 | 83280 | 90880 | 100700 | 109100 | 117800 | 129900 | 137530 | 602930 | |||||||||
| CRC | 6.8281 | 13.5755 | 27.4917 | 43.2672 | 99.30 | 119.53 | 140.8 | 170.4 | 192.1 | 215.92 | 265.07 | 291.500 | 787.84 | 863.1 | 941.9 | 1044 | 1131 | 1221 | 1346 | 1425.4 | 6249.0 | 6625.82 | ||||||||
| 23 V vanadium | ||||||||||||||||||||||||||||||
| use | 650.9 | 1414 | 2830 | 4507 | 6298.7 | 12363 | 14530 | 16730 | 19860 | 22240 | 24670 | 29730 | 32446 | 86450 | 94170 | 102300 | 112700 | 121600 | 130700 | 143400 | 151440 | 661050 | 699144 | |||||||
| WEL | 650.9 | 1414 | 2830 | 4507 | 6298.7 | 12363 | 14530 | 16730 | 19860 | 22240 | 24670 | 29730 | 32446 | 86450 | 94170 | 102300 | 112700 | 121600 | 130700 | 143400 | 151440 | |||||||||
| CRC | 6.7462 | 14.66 | 29.311 | 46.709 | 65.2817 | 128.13 | 150.6 | 173.4 | 205.8 | 230.5 | 255.7 | 308.1 | 336.277 | 896.0 | 976 | 1060 | 1168 | 1260 | 1355 | 1486 | 1569.6 | 6851.3 | 7246.12 | |||||||
| 24 Cr chromium | ||||||||||||||||||||||||||||||
| use | 652.9 | 1590.6 | 2987 | 4743 | 6702 | 8744.9 | 15455 | 17820 | 20190 | 23580 | 26130 | 28750 | 34230 | 37066 | 97510 | 105800 | 114300 | 125300 | 134700 | 144300 | 157700 | 166090 | 721870 | 761733 | ||||||
| WEL | 652.9 | 1590.6 | 2987 | 4743 | 6702 | 8744.9 | 15455 | 17820 | 20190 | 23580 | 26130 | 28750 | 34230 | 37066 | 97510 | 105800 | 114300 | 125300 | 134700 | 144300 | 157700 | |||||||||
| CRC | 6.7665 | 16.4857 | 30.96 | 49.16 | 69.46 | 90.6349 | 160.18 | 184.7 | 209.3 | 244.4 | 270.8 | 298.0 | 354.8 | 384.168 | 1010.6 | 1097 | 1185 | 1299 | 1396 | 1496 | 1634 | 1721.4 | 7481.7 | 7894.81 | ||||||
| 25 Mn manganese | ||||||||||||||||||||||||||||||
| use | 717.3 | 1509.0 | 3248 | 4940 | 6990 | 9220 | 11500 | 18770 | 21400 | 23960 | 27590 | 30330 | 33150 | 38880 | 41987 | 109480 | 118100 | 127100 | 138600 | 148500 | 158600 | 172500 | 181380 | 785450 | 827067 | |||||
| WEL | 717.3 | 1509.0 | 3248 | 4940 | 6990 | 9220 | 11500 | 18770 | 21400 | 23960 | 27590 | 30330 | 33150 | 38880 | 41987 | 109480 | 118100 | 127100 | 138600 | 148500 | 158600 | |||||||||
| CRC | 7.43402 | 15.63999 | 33.668 | 51.2 | 72.4 | 95.6 | 119.203 | 194.5 | 221.8 | 248.3 | 286.0 | 314.4 | 343.6 | 403.0 | 435.163 | 1134.7 | 1224 | 1317 | 1437 | 1539 | 1644 | 1788 | 1879.9 | 8140.6 | 8571.94 | |||||
| 26 Fe iron | ||||||||||||||||||||||||||||||
| use | 762.5 | 1561.9 | 2957 | 5290 | 7240 | 9560 | 12060 | 14580 | 22540 | 25290 | 28000 | 31920 | 34830 | 37840 | 44100 | 47206 | 122200 | 131000 | 140500 | 152600 | 163000 | 173600 | 188100 | 195200 | 851800 | 895161 | ||||
| WEL | 762.5 | 1561.9 | 2957 | 5290 | 7240 | 9560 | 12060 | 14580 | 22540 | 25290 | 28000 | 31920 | 34830 | 37840 | 44100 | 47206 | 122200 | 131000 | 140500 | 152600 | 163000 | |||||||||
| CRC | 7.9024 | 16.1878 | 30.652 | 54.8 | 75.0 | 99.1 | 124.98 | 151.06 | 233.6 | 262.1 | 290.2 | 330.8 | 361.0 | 392.2 | 457 | 489.256 | 1266 | 1358 | 1456 | 1582 | 1689 | 1799 | 1950 | 2023 | 8828 | 9277.69 | ||||
| 27 Co cobalt | ||||||||||||||||||||||||||||||
| use | 760.4 | 1648 | 3232 | 4950 | 7670 | 9840 | 12440 | 15230 | 17959 | 26570 | 29400 | 32400 | 36600 | 39700 | 42800 | 49396 | 52737 | 134810 | 145170 | 154700 | 167400 | 178100 | 189300 | 204500 | 214100 | 920870 | 966023 | |||
| WEL | 760.4 | 1648 | 3232 | 4950 | 7670 | 9840 | 12440 | 15230 | 17959 | 26570 | 29400 | 32400 | 36600 | 39700 | 42800 | 49396 | 52737 | 134810 | 145170 | 154700 | 167400 | |||||||||
| CRC | 7.8810 | 17.083 | 33.50 | 51.3 | 79.5 | 102.0 | 128.9 | 157.8 | 186.13 | 275.4 | 305 | 336 | 379 | 411 | 444 | 511.96 | 546.58 | 1397.2 | 1504.6 | 1603 | 1735 | 1846 | 1962 | 2119 | 2219.0 | 9544.1 | 10012.12 | |||
| 28 Ni nickel | ||||||||||||||||||||||||||||||
| use | 737.1 | 1753.0 | 3395 | 5300 | 7339 | 10400 | 12800 | 15600 | 18600 | 21670 | 30970 | 34000 | 37100 | 41500 | 44800 | 48100 | 55101 | 58570 | 148700 | 159000 | 169400 | 182700 | 194000 | 205600 | 221400 | 231490 | 992718 | 1039668 | ||
| WEL | 737.1 | 1753.0 | 3395 | 5300 | 7339 | 10400 | 12800 | 15600 | 18600 | 21670 | 30970 | 34000 | 37100 | 41500 | 44800 | 48100 | 55101 | 58570 | 148700 | 159000 | 169400 | |||||||||
| CRC | 7.6398 | 18.16884 | 35.19 | 54.9 | 76.06 | 108 | 133 | 162 | 193 | 224.6 | 321.0 | 352 | 384 | 430 | 464 | 499 | 571.08 | 607.06 | 1541 | 1648 | 1756 | 1894 | 2011 | 2131 | 2295 | 2399.2 | 10288.8 | 10775.40 | ||
| 29 Cu copper | ||||||||||||||||||||||||||||||
| use | 745.5 | 1957.9 | 3555 | 5536 | 7700 | 9900 | 13400 | 16000 | 19200 | 22400 | 25600 | 35600 | 38700 | 42000 | 46700 | 50200 | 53700 | 61100 | 64702 | 163700 | 174100 | 184900 | 198800 | 210500 | 222700 | 239100 | 249660 | 1067358 | 1116105 | |
| WEL | 745.5 | 1957.9 | 3555 | 5536 | 7700 | 9900 | 13400 | 16000 | 19200 | 22400 | 25600 | 35600 | 38700 | 42000 | 46700 | 50200 | 53700 | 61100 | 64702 | 163700 | 174100 | |||||||||
| CRC | 7.72638 | 20.29240 | 36.841 | 57.38 | 79.8 | 103 | 139 | 166 | 199 | 232 | 265.3 | 369 | 401 | 435 | 484 | 520 | 557 | 633 | 670.588 | 1697 | 1804 | 1916 | 2060 | 2182 | 2308 | 2478 | 2587.5 | 11062.38 | 11567.617 | |
| 30 Zn zinc | ||||||||||||||||||||||||||||||
| use | 906.4 | 1733.3 | 3833 | 5731 | 7970 | 10400 | 12900 | 16800 | 19600 | 23000 | 26400 | 29990 | 40490 | 43800 | 47300 | 52300 | 55900 | 59700 | 67300 | 71200 | 179100 | |||||||||
| WEL | 906.4 | 1733.3 | 3833 | 5731 | 7970 | 10400 | 12900 | 16800 | 19600 | 23000 | 26400 | 29990 | 40490 | 43800 | 47300 | 52300 | 55900 | 59700 | 67300 | 71200 | 179100 | |||||||||
| CRC | 9.3942 | 17.96440 | 39.723 | 59.4 | 82.6 | 108 | 134 | 174 | 203 | 238 | 274 | 310.8 | 419.7 | 454 | 490 | 542 | 579 | 619 | 698 | 738 | 1856 | |||||||||
| 31 Ga gallium | ||||||||||||||||||||||||||||||
| use | 578.8 | 1979.3 | 2963 | 6180 | ||||||||||||||||||||||||||
| WEL | 578.8 | 1979.3 | 2963 | 6180 | ||||||||||||||||||||||||||
| CRC | 5.99930 | 20.5142 | 30.71 | 64 | ||||||||||||||||||||||||||
| 32 Ge germanium | ||||||||||||||||||||||||||||||
| use | 762 | 1537.5 | 3302.1 | 4411 | 9020 | |||||||||||||||||||||||||
| WEL | 762 | 1537.5 | 3302.1 | 4411 | 9020 | |||||||||||||||||||||||||
| CRC | 7.8994 | 15.93462 | 34.2241 | 45.7131 | 93.5 | |||||||||||||||||||||||||
| 33 As arsenic | ||||||||||||||||||||||||||||||
| use | 947.0 | 1798 | 2735 | 4837 | 6043 | 12310 | ||||||||||||||||||||||||
| WEL | 947.0 | 1798 | 2735 | 4837 | 6043 | 12310 | ||||||||||||||||||||||||
| CRC | 9.7886 | 18.633 | 28.351 | 50.13 | 62.63 | 127.6 | ||||||||||||||||||||||||
| 34 Se selenium | ||||||||||||||||||||||||||||||
| use | 941.0 | 2045 | 2973.7 | 4144 | 6590 | 7880 | 14990 | |||||||||||||||||||||||
| WEL | 941.0 | 2045 | 2973.7 | 4144 | 6590 | 7880 | 14990 | |||||||||||||||||||||||
| CRC | 9.75238 | 21.19 | 30.8204 | 42.9450 | 68.3 | 81.7 | 155.4 | |||||||||||||||||||||||
| 35 Br bromine | ||||||||||||||||||||||||||||||
| use | 1139.9 | 2103 | 3470 | 4560 | 5760 | 8550 | 9940 | 18600 | ||||||||||||||||||||||
| WEL | 1139.9 | 2103 | 3470 | 4560 | 5760 | 8550 | 9940 | 18600 | ||||||||||||||||||||||
| CRC | 11.81381 | 21.8 | 36 | 47.3 | 59.7 | 88.6 | 103.0 | 192.8 | ||||||||||||||||||||||
| 36 Kr krypton | ||||||||||||||||||||||||||||||
| use | 1350.8 | 2350.4 | 3565 | 5070 | 6240 | 7570 | 10710 | 12138 | 22274 | 25880 | 29700 | 33800 | 37700 | 43100 | 47500 | 52200 | 57100 | 61800 | 75800 | 80400 | 85300 | 90400 | 96300 | 101400 | 111100 | 116290 | 282500 | 296200 | 311400 | 326200 |
| WEL | 1350.8 | 2350.4 | 3565 | 5070 | 6240 | 7570 | 10710 | 12138 | 22274 | 25880 | 29700 | 33800 | 37700 | 43100 | 47500 | 52200 | 57100 | 61800 | 75800 | 80400 | 85300 | |||||||||
| CRC | 13.99961 | 24.35985 | 36.950 | 52.5 | 64.7 | 78.5 | 111.0 | 125.802 | 230.85 | 268.2 | 308 | 350 | 391 | 447 | 492 | 541 | 592 | 641 | 786 | 833 | 884 | 937 | 998 | 1051 | 1151 | 1205.3 | 2928 | 3070 | 3227 | 3381 |
| 37 Rb rubidium | ||||||||||||||||||||||||||||||
| use | 403.0 | 2633 | 3860 | 5080 | 6850 | 8140 | 9570 | 13120 | 14500 | 26740 | ||||||||||||||||||||
| WEL | 403.0 | 2633 | 3860 | 5080 | 6850 | 8140 | 9570 | 13120 | 14500 | 26740 | ||||||||||||||||||||
| CRC | 4.17713 | 27.285 | 40 | 52.6 | 71.0 | 84.4 | 99.2 | 136 | 150 | 277.1 | ||||||||||||||||||||
| 38 Sr strontium | ||||||||||||||||||||||||||||||
| use | 549.5 | 1064.2 | 4138 | 5500 | 6910 | 8760 | 10230 | 11800 | 15600 | 17100 | 31270 | |||||||||||||||||||
| WEL | 549.5 | 1064.2 | 4138 | 5500 | 6910 | 8760 | 10230 | 11800 | 15600 | 17100 | ||||||||||||||||||||
| CRC | 5.6949 | 11.03013 | 42.89 | 57 | 71.6 | 90.8 | 106 | 122.3 | 162 | 177 | 324.1 | |||||||||||||||||||
| 39 Y yttrium | ||||||||||||||||||||||||||||||
| use | 600 | 1180 | 1980 | 5847 | 7430 | 8970 | 11190 | 12450 | 14110 | 18400 | 19900 | 36090 | ||||||||||||||||||
| WEL | 600 | 1180 | 1980 | 5847 | 7430 | 8970 | 11190 | 12450 | 14110 | 18400 | ||||||||||||||||||||
| CRC | 6.2171 | 12.24 | 20.52 | 60.597 | 77.0 | 93.0 | 116 | 129 | 146.2 | 191 | 206 | 374.0 | ||||||||||||||||||
| 40 Zr zirconium | ||||||||||||||||||||||||||||||
| use | 640.1 | 1270 | 2218 | 3313 | 7752 | 9500 | ||||||||||||||||||||||||
| WEL | 640.1 | 1270 | 2218 | 3313 | 7752 | 9500 | ||||||||||||||||||||||||
| CRC | 6.63390 | 13.13 | 22.99 | 34.34 | 80.348 | |||||||||||||||||||||||||
| 41 Nb niobium | ||||||||||||||||||||||||||||||
| use | 652.1 | 1380 | 2416 | 3700 | 4877 | 9847 | 12100 | |||||||||||||||||||||||
| WEL | 652.1 | 1380 | 2416 | 3700 | 4877 | 9847 | 12100 | |||||||||||||||||||||||
| CRC | 6.75885 | 14.32 | 25.04 | 38.3 | 50.55 | 102.057 | 125 | |||||||||||||||||||||||
| 42 Mo molybdenum | ||||||||||||||||||||||||||||||
| use | 684.3 | 1560 | 2618 | 4480 | 5257 | 6640.8 | 12125 | 13860 | 15835 | 17980 | 20190 | 22219 | 26930 | 29196 | 52490 | 55000 | 61400 | 67700 | 74000 | 80400 | 87000 | 93400 | 98420 | 104400 | 121900 | 127700 | 133800 | 139800 | 148100 | 154500 |
| WEL | 684.3 | 1560 | 2618 | 4480 | 5257 | 6640.8 | 12125 | 13860 | 15835 | 17980 | 20190 | 22219 | 26930 | 29196 | 52490 | 55000 | 61400 | 67700 | 74000 | 80400 | 87000 | |||||||||
| CRC | 7.09243 | 16.16 | 27.13 | 46.4 | 54.49 | 68.8276 | 125.664 | 143.6 | 164.12 | 186.4 | 209.3 | 230.28 | 279.1 | 302.60 | 544.0 | 570 | 636 | 702 | 767 | 833 | 902 | 968 | 1020 | 1082 | 1263 | 1323 | 1387 | 1449 | 1535 | 1601 |
| 43 Tc technetium | ||||||||||||||||||||||||||||||
| use | 686.9 | 1470 | 2850 | |||||||||||||||||||||||||||
| WEL | 702 | 1470 | 2850 | |||||||||||||||||||||||||||
| CRC | 7.28 | 15.26 | 29.54 | |||||||||||||||||||||||||||
| Mattolat et al. [10] | 7.119380(32) eV | |||||||||||||||||||||||||||||
| 44 Ru ruthenium | ||||||||||||||||||||||||||||||
| use | 710.2 | 1620 | 2747 | |||||||||||||||||||||||||||
| WEL | 710.2 | 1620 | 2747 | |||||||||||||||||||||||||||
| CRC | 7.36050 | 16.76 | 28.47 | |||||||||||||||||||||||||||
| 45 Rh rhodium | ||||||||||||||||||||||||||||||
| use | 719.7 | 1740 | 2997 | |||||||||||||||||||||||||||
| WEL | 719.7 | 1740 | 2997 | |||||||||||||||||||||||||||
| CRC | 7.45890 | 18.08 | 31.06 | |||||||||||||||||||||||||||
| 46 Pd palladium | ||||||||||||||||||||||||||||||
| use | 804.4 | 1870 | 3177 | |||||||||||||||||||||||||||
| WEL | 804.4 | 1870 | 3177 | |||||||||||||||||||||||||||
| CRC | 8.3369 | 19.43 | 32.93 | |||||||||||||||||||||||||||
| 47 Ag silver | ||||||||||||||||||||||||||||||
| use | 731.0 | 2070 | 3361 | |||||||||||||||||||||||||||
| WEL | 731.0 | 2070 | 3361 | |||||||||||||||||||||||||||
| CRC | 7.5762 | 21.49 | 34.83 | |||||||||||||||||||||||||||
| 48 Cd cadmium | ||||||||||||||||||||||||||||||
| use | 867.8 | 1631.4 | 3616 | |||||||||||||||||||||||||||
| WEL | 867.8 | 1631.4 | 3616 | |||||||||||||||||||||||||||
| CRC | 8.9938 | 16.90832 | 37.48 | |||||||||||||||||||||||||||
| 49 In indium | ||||||||||||||||||||||||||||||
| use | 558.3 | 1820.7 | 2704 | 5210 | ||||||||||||||||||||||||||
| WEL | 558.3 | 1820.7 | 2704 | 5210 | ||||||||||||||||||||||||||
| CRC | 5.78636 | 18.8698 | 28.03 | 54 | ||||||||||||||||||||||||||
| 50 Sn tin | ||||||||||||||||||||||||||||||
| use | 708.6 | 1411.8 | 2943.0 | 3930.3 | 7456 | |||||||||||||||||||||||||
| WEL | 708.6 | 1411.8 | 2943.0 | 3930.3 | 7456 | |||||||||||||||||||||||||
| CRC | 7.3439 | 14.63225 | 30.50260 | 40.73502 | 72.28 | |||||||||||||||||||||||||
| 51 Sb antimony | ||||||||||||||||||||||||||||||
| use | 834 | 1594.9 | 2440 | 4260 | 5400 | 10400 | ||||||||||||||||||||||||
| WEL | 834 | 1594.9 | 2440 | 4260 | 5400 | 10400 | ||||||||||||||||||||||||
| CRC | 8.6084 | 16.53051 | 25.3 | 44.2 | 56 | 108 | ||||||||||||||||||||||||
| 52 Te tellurium | ||||||||||||||||||||||||||||||
| use | 869.3 | 1790 | 2698 | 3610 | 5668 | 6820 | 13200 | |||||||||||||||||||||||
| WEL | 869.3 | 1790 | 2698 | 3610 | 5668 | 6820 | 13200 | |||||||||||||||||||||||
| CRC | 9.0096 | 18.6 | 27.96 | 37.41 | 58.75 | 70.7 | 137 | |||||||||||||||||||||||
| 53 I iodine | ||||||||||||||||||||||||||||||
| use | 1008.4 | 1845.9 | 3180 | |||||||||||||||||||||||||||
| WEL | 1008.4 | 1845.9 | 3180 | |||||||||||||||||||||||||||
| CRC | 10.45126 | 19.1313 | 33 | |||||||||||||||||||||||||||
| 54 Xe xenon | ||||||||||||||||||||||||||||||
| use | 1170.4 | 2046.4 | 3099.4 | |||||||||||||||||||||||||||
| WEL | 1170.4 | 2046.4 | 3099.4 | |||||||||||||||||||||||||||
| CRC | 12.1298 | 21.20979 | 32.1230 | |||||||||||||||||||||||||||
| 55 Cs caesium | ||||||||||||||||||||||||||||||
| use | 375.7 | 2234.3 | 3400 | |||||||||||||||||||||||||||
| WEL | 375.7 | 2234.3 | 3400 | |||||||||||||||||||||||||||
| CRC | 3.89390 | 23.15745 | ||||||||||||||||||||||||||||
| 56 Ba barium | ||||||||||||||||||||||||||||||
| use | 502.9 | 965.2 | 3600 | |||||||||||||||||||||||||||
| WEL | 502.9 | 965.2 | 3600 | |||||||||||||||||||||||||||
| CRC | 5.21170 | 10.00390 | ||||||||||||||||||||||||||||
| 57 La lanthanum | ||||||||||||||||||||||||||||||
| use | 538.1 | 1067 | 1850.3 | 4819 | 5940 | |||||||||||||||||||||||||
| WEL | 538.1 | 1067 | 1850.3 | 4819 | 5940 | |||||||||||||||||||||||||
| CRC | 5.5769 | 11.060 | 19.1773 | 49.95 | 61.6 | |||||||||||||||||||||||||
| 58 Ce cerium | ||||||||||||||||||||||||||||||
| use | 534.4 | 1050 | 1949 | 3547 | 6325 | 7490 | ||||||||||||||||||||||||
| WEL | 534.4 | 1050 | 1949 | 3547 | 6325 | 7490 | ||||||||||||||||||||||||
| CRC | 5.5387 | 10.85 | 20.198 | 36.758 | 65.55 | 77.6 | ||||||||||||||||||||||||
| 59 Pr praseodymium | ||||||||||||||||||||||||||||||
| use | 527 | 1020 | 2086 | 3761 | 5551 | |||||||||||||||||||||||||
| WEL | 527 | 1020 | 2086 | 3761 | 5551 | |||||||||||||||||||||||||
| CRC | 5.473 | 10.55 | 21.624 | 38.98 | 57.53 | |||||||||||||||||||||||||
| 60 Nd neodymium | ||||||||||||||||||||||||||||||
| use | 533.1 | 1040 | 2130 | 3900 | ||||||||||||||||||||||||||
| WEL | 533.1 | 1040 | 2130 | 3900 | ||||||||||||||||||||||||||
| CRC | 5.5250 | 10.73 | 22.1 | 40.41 | ||||||||||||||||||||||||||
| 61 Pm promethium | ||||||||||||||||||||||||||||||
| use | 540 | 1050 | 2150 | 3970 | ||||||||||||||||||||||||||
| WEL | 540 | 1050 | 2150 | 3970 | ||||||||||||||||||||||||||
| CRC | 5.582 | 10.90 | 22.3 | 41.1 | ||||||||||||||||||||||||||
| 62 Sm samarium | ||||||||||||||||||||||||||||||
| use | 544.5 | 1070 | 2260 | 3990 | ||||||||||||||||||||||||||
| WEL | 544.5 | 1070 | 2260 | 3990 | ||||||||||||||||||||||||||
| CRC | 5.6436 | 11.07 | 23.4 | 41.4 | ||||||||||||||||||||||||||
| 63 Eu europium | ||||||||||||||||||||||||||||||
| use | 547.1 | 1085 | 2404 | 4120 | ||||||||||||||||||||||||||
| WEL | 547.1 | 1085 | 2404 | 4120 | ||||||||||||||||||||||||||
| CRC | 5.6704 | 11.241 | 24.92 | 42.7 | ||||||||||||||||||||||||||
| 64 Gd gadolinium | ||||||||||||||||||||||||||||||
| use | 593.4 | 1170 | 1990 | 4250 | ||||||||||||||||||||||||||
| WEL | 593.4 | 1170 | 1990 | 4250 | ||||||||||||||||||||||||||
| CRC | 6.1501 | 12.09 | 20.63 | 44.0 | ||||||||||||||||||||||||||
| 65 Tb terbium | ||||||||||||||||||||||||||||||
| use | 565.8 | 1110 | 2114 | 3839 | ||||||||||||||||||||||||||
| WEL | 565.8 | 1110 | 2114 | 3839 | ||||||||||||||||||||||||||
| CRC | 5.8638 | 11.52 | 21.91 | 39.79 | ||||||||||||||||||||||||||
| 66 Dy dysprosium | ||||||||||||||||||||||||||||||
| use | 573.0 | 1130 | 2200 | 3990 | ||||||||||||||||||||||||||
| WEL | 573.0 | 1130 | 2200 | 3990 | ||||||||||||||||||||||||||
| CRC | 5.9389 | 11.67 | 22.8 | 41.47 | ||||||||||||||||||||||||||
| 67 Ho holmium | ||||||||||||||||||||||||||||||
| use | 581.0 | 1140 | 2204 | 4100 | ||||||||||||||||||||||||||
| WEL | 581.0 | 1140 | 2204 | 4100 | ||||||||||||||||||||||||||
| CRC | 6.0215 | 11.80 | 22.84 | 42.5 | ||||||||||||||||||||||||||
| 68 Er erbium | ||||||||||||||||||||||||||||||
| use | 589.3 | 1150 | 2194 | 4120 | ||||||||||||||||||||||||||
| WEL | 589.3 | 1150 | 2194 | 4120 | ||||||||||||||||||||||||||
| CRC | 6.1077 | 11.93 | 22.74 | 42.7 | ||||||||||||||||||||||||||
| 69 Tm thulium | ||||||||||||||||||||||||||||||
| use | 596.7 | 1160 | 2285 | 4120 | ||||||||||||||||||||||||||
| WEL | 596.7 | 1160 | 2285 | 4120 | ||||||||||||||||||||||||||
| CRC | 6.18431 | 12.05 | 23.68 | 42.7 | ||||||||||||||||||||||||||
| 70 Yb ytterbium | ||||||||||||||||||||||||||||||
| use | 603.4 | 1174.8 | 2417 | 4203 | ||||||||||||||||||||||||||
| WEL | 603.4 | 1174.8 | 2417 | 4203 | ||||||||||||||||||||||||||
| CRC | 6.25416 | 12.1761 | 25.05 | 43.56 | ||||||||||||||||||||||||||
| 71 Lu lutetium | ||||||||||||||||||||||||||||||
| use | 523.5 | 1340 | 2022.3 | 4370 | 6445 | |||||||||||||||||||||||||
| WEL | 523.5 | 1340 | 2022.3 | 4370 | 6445 | |||||||||||||||||||||||||
| CRC | 5.4259 | 13.9 | 20.9594 | 45.25 | 66.8 | |||||||||||||||||||||||||
| 72 Hf hafnium | ||||||||||||||||||||||||||||||
| use | 658.5 | 1440 | 2250 | 3216 | ||||||||||||||||||||||||||
| WEL | 658.5 | 1440 | 2250 | 3216 | ||||||||||||||||||||||||||
| CRC | 6.82507 | 14.9 | 23.3 | 33.33 | ||||||||||||||||||||||||||
| 73 Ta tantalum | ||||||||||||||||||||||||||||||
| use | 761 | 1500 | ||||||||||||||||||||||||||||
| WEL | 761 | 1500 | ||||||||||||||||||||||||||||
| CRC | 7.5496 | |||||||||||||||||||||||||||||
| 74 W tungsten | ||||||||||||||||||||||||||||||
| use | 770 | 1700 | ||||||||||||||||||||||||||||
| WEL | 770 | 1700 | ||||||||||||||||||||||||||||
| CRC | 7.8640 | |||||||||||||||||||||||||||||
| 75 Re rhenium | ||||||||||||||||||||||||||||||
| use | 760 | 1260 | 2510 | 3640 | ||||||||||||||||||||||||||
| WEL | 760 | 1260 | 2510 | 3640 | ||||||||||||||||||||||||||
| CRC | 7.8335 | |||||||||||||||||||||||||||||
| 76 Os osmium | ||||||||||||||||||||||||||||||
| use | 840 | 1600 | ||||||||||||||||||||||||||||
| WEL | 840 | 1600 | ||||||||||||||||||||||||||||
| CRC | 8.4382 | |||||||||||||||||||||||||||||
| 77 Ir iridium | ||||||||||||||||||||||||||||||
| use | 880 | 1600 | ||||||||||||||||||||||||||||
| WEL | 880 | 1600 | ||||||||||||||||||||||||||||
| CRC | 8.9670 | |||||||||||||||||||||||||||||
| 78 Pt platinum | ||||||||||||||||||||||||||||||
| use | 870 | 1791 | ||||||||||||||||||||||||||||
| WEL | 870 | 1791 | ||||||||||||||||||||||||||||
| CRC | 8.9587 | 18.563 | 28 [11] | |||||||||||||||||||||||||||
| 79 Au gold | ||||||||||||||||||||||||||||||
| use | 890.1 | 1980 | ||||||||||||||||||||||||||||
| WEL | 890.1 | 1980 | ||||||||||||||||||||||||||||
| CRC | 9.2255 | 20.5 | 30 [11] | |||||||||||||||||||||||||||
| 80 Hg mercury | ||||||||||||||||||||||||||||||
| use | 1007.1 | 1810 | 3300 | |||||||||||||||||||||||||||
| WEL | 1007.1 | 1810 | 3300 | |||||||||||||||||||||||||||
| CRC | 10.43750 | 18.756 | 34.2 | |||||||||||||||||||||||||||
| 81 Tl thallium | ||||||||||||||||||||||||||||||
| use | 589.4 | 1971 | 2878 | |||||||||||||||||||||||||||
| WEL | 589.4 | 1971 | 2878 | |||||||||||||||||||||||||||
| CRC | 6.1082 | 20.428 | 29.83 | |||||||||||||||||||||||||||
| 82 Pb lead | ||||||||||||||||||||||||||||||
| use | 715.6 | 1450.5 | 3081.5 | 4083 | 6640 | |||||||||||||||||||||||||
| WEL | 715.6 | 1450.5 | 3081.5 | 4083 | 6640 | |||||||||||||||||||||||||
| CRC | 7.41666 | 15.0322 | 31.9373 | 42.32 | 68.8 | |||||||||||||||||||||||||
| 83 Bi bismuth | ||||||||||||||||||||||||||||||
| use | 703 | 1610 | 2466 | 4370 | 5400 | 8520 | ||||||||||||||||||||||||
| WEL | 703 | 1610 | 2466 | 4370 | 5400 | 8520 | ||||||||||||||||||||||||
| CRC | 7.2856 | 16.69 | 25.56 | 45.3 | 56.0 | 88.3 | ||||||||||||||||||||||||
| 84 Po polonium | ||||||||||||||||||||||||||||||
| use | 812.1 | |||||||||||||||||||||||||||||
| WEL | 812.1 | |||||||||||||||||||||||||||||
| CRC | 8.417 | |||||||||||||||||||||||||||||
| 85 At astatine | ||||||||||||||||||||||||||||||
| use | 899.003 | |||||||||||||||||||||||||||||
| WEL | 920 | |||||||||||||||||||||||||||||
| CRC | ||||||||||||||||||||||||||||||
| talk | Originally quoted as 9.31751(8) eV. | |||||||||||||||||||||||||||||
| 86 Rn radon | ||||||||||||||||||||||||||||||
| use | 1037 | |||||||||||||||||||||||||||||
| WEL | 1037 | |||||||||||||||||||||||||||||
| CRC | 10.74850 | |||||||||||||||||||||||||||||
| 87 Fr francium | ||||||||||||||||||||||||||||||
| use | 380 | |||||||||||||||||||||||||||||
| WEL | 380 | |||||||||||||||||||||||||||||
| CRC | 4.0727 | |||||||||||||||||||||||||||||
| talk | Andreev, S.V.; Letokhov, V.S.; Mishin, V.I. (1987). "Laser resonance photoionization spectroscopy of Rydberg levels in Fr". Phys. Rev. Lett. 59 (12): 1274–76. Bibcode:1987PhRvL..59.1274A. doi:10.1103/PhysRevLett.59.1274. PMID 10035190. give 4.0712±0.00004 eV (392.811(4) kJ/mol) | |||||||||||||||||||||||||||||
| 88 Ra radium | ||||||||||||||||||||||||||||||
| use | 509.3 | 979.0 | ||||||||||||||||||||||||||||
| WEL | 509.3 | 979.0 | ||||||||||||||||||||||||||||
| CRC | 5.2784 | 10.14716 | ||||||||||||||||||||||||||||
| 89 Ac actinium | ||||||||||||||||||||||||||||||
| use | 499 | 1170 | ||||||||||||||||||||||||||||
| WEL | 499 | 1170 | ||||||||||||||||||||||||||||
| CRC | 5.17 | 12.1 | ||||||||||||||||||||||||||||
| 90 Th thorium | ||||||||||||||||||||||||||||||
| use | 587 | 1110 | 1930 | 2780 | ||||||||||||||||||||||||||
| WEL | 587 | 1110 | 1930 | 2780 | ||||||||||||||||||||||||||
| CRC | 6.3067 | 11.5 | 20.0 | 28.8 | ||||||||||||||||||||||||||
| 91 Pa protactinium | ||||||||||||||||||||||||||||||
| use | 568 | |||||||||||||||||||||||||||||
| WEL | 568 | |||||||||||||||||||||||||||||
| CRC | 5.89 | |||||||||||||||||||||||||||||
| 92 U uranium | ||||||||||||||||||||||||||||||
| use | 597.6 | 1420 | ||||||||||||||||||||||||||||
| WEL | 597.6 | 1420 | ||||||||||||||||||||||||||||
| CRC | 6.19405 | 14.72 | ||||||||||||||||||||||||||||
| 93 Np neptunium | ||||||||||||||||||||||||||||||
| use | 604.5 | |||||||||||||||||||||||||||||
| WEL | 604.5 | |||||||||||||||||||||||||||||
| CRC | 6.2657 | |||||||||||||||||||||||||||||
| 94 Pu plutonium | ||||||||||||||||||||||||||||||
| use | 584.7 | |||||||||||||||||||||||||||||
| WEL | 584.7 | |||||||||||||||||||||||||||||
| CRC | 6.0262 | |||||||||||||||||||||||||||||
| 95 Am americium | ||||||||||||||||||||||||||||||
| use | 578 | |||||||||||||||||||||||||||||
| WEL | 578 | |||||||||||||||||||||||||||||
| CRC | 5.9738 | |||||||||||||||||||||||||||||
| 96 Cm curium | ||||||||||||||||||||||||||||||
| use | 581 | |||||||||||||||||||||||||||||
| WEL | 581 | |||||||||||||||||||||||||||||
| CRC | 5.9915 | |||||||||||||||||||||||||||||
| 97 Bk berkelium | ||||||||||||||||||||||||||||||
| use | 601 | |||||||||||||||||||||||||||||
| WEL | 601 | |||||||||||||||||||||||||||||
| CRC | 6.1979 | |||||||||||||||||||||||||||||
| 98 Cf californium | ||||||||||||||||||||||||||||||
| use | 608 | |||||||||||||||||||||||||||||
| WEL | 608 | |||||||||||||||||||||||||||||
| CRC | 6.2817 | |||||||||||||||||||||||||||||
| 99 Es einsteinium | ||||||||||||||||||||||||||||||
| use | 619 | |||||||||||||||||||||||||||||
| WEL | 619 | |||||||||||||||||||||||||||||
| CRC | 6.42 | |||||||||||||||||||||||||||||
| 100 Fm fermium | ||||||||||||||||||||||||||||||
| use | 627 | |||||||||||||||||||||||||||||
| WEL | 627 | |||||||||||||||||||||||||||||
| CRC | 6.50 | |||||||||||||||||||||||||||||
| 101 Md mendelevium | ||||||||||||||||||||||||||||||
| use | 635 | |||||||||||||||||||||||||||||
| WEL | 635 | |||||||||||||||||||||||||||||
| CRC | 6.58 | |||||||||||||||||||||||||||||
| 102 No nobelium | ||||||||||||||||||||||||||||||
| use | 642 | |||||||||||||||||||||||||||||
| WEL | ||||||||||||||||||||||||||||||
| CRC | 6.65 | |||||||||||||||||||||||||||||
| 103 Lr lawrencium | ||||||||||||||||||||||||||||||
| use | 470 | |||||||||||||||||||||||||||||
| WEL | ||||||||||||||||||||||||||||||
| CRC | 4.9 | |||||||||||||||||||||||||||||
| 104 Rf rutherfordium | ||||||||||||||||||||||||||||||
| use | 580 | |||||||||||||||||||||||||||||
| WEL | ||||||||||||||||||||||||||||||
| CRC | 6.0 | |||||||||||||||||||||||||||||
The molecular mass (m) is the mass of a given molecule. The unit dalton (Da) is often used. Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The derived quantity relative molecular mass is the unitless ratio of the mass of a molecule to the atomic mass constant (which is equal to one dalton).

The Avogadro constant, commonly denoted NA or L, is an SI defining constant with an exact value of 6.02214076×1023 mol−1 (reciprocal moles). It is defined as the number of constituent particles (usually molecules, atoms, ions, or ion pairs) per mole (SI unit) and used as a normalization factor in the amount of substance in a sample. In the SI dimensional analysis of measurement units, the dimension of the Avogadro constant is the reciprocal of amount of substance, denoted N−1. The Avogadro number, sometimes denoted N0, is the numeric value of the Avogadro constant (i.e., without a unit), namely the dimensionless number 6.02214076×1023; the value chosen based on the number of atoms in 12 grams of carbon-12 in alignment with the historical definition of a mole. The constant is named after the Italian physicist and chemist Amedeo Avogadro (1776–1856).
The dalton or unified atomic mass unit is a unit of mass defined as 1/12 of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. It is a non-SI unit accepted for use with SI. The atomic mass constant, denoted mu, is defined identically, giving mu = 1/12m(12C) = 1 Da.

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules, electrons, positrons, protons, antiprotons and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.

In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. The first ionization energy is quantitatively expressed as
In spectroscopy, the Rydberg constant, symbol for heavy atoms or for hydrogen, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to the electromagnetic spectra of an atom. The constant first arose as an empirical fitting parameter in the Rydberg formula for the hydrogen spectral series, but Niels Bohr later showed that its value could be calculated from more fundamental constants according to his model of the atom.
The Balmer series, or Balmer lines in atomic physics, is one of a set of six named series describing the spectral line emissions of the hydrogen atom. The Balmer series is calculated using the Balmer formula, an empirical equation discovered by Johann Balmer in 1885.

Photoionization is the physical process in which an ion is formed from the interaction of a photon with an atom or molecule.
The molar heat capacity of a chemical substance is the amount of energy that must be added, in the form of heat, to one mole of the substance in order to cause an increase of one unit in its temperature. Alternatively, it is the heat capacity of a sample of the substance divided by the amount of substance of the sample; or also the specific heat capacity of the substance times its molar mass. The SI unit of molar heat capacity is joule per kelvin per mole, J⋅K−1⋅mol−1.
Spectroscopic notation provides a way to specify atomic ionization states, atomic orbitals, and molecular orbitals.
In chemistry, bond energy (BE) is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy for all bonds of the same type within the same chemical species.
These tables list values of molar ionization energies, measured in kJ⋅mol−1. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. The first molar ionization energy applies to the neutral atoms. The second, third, etc., molar ionization energy applies to the further removal of an electron from a singly, doubly, etc., charged ion. For ionization energies measured in the unit eV, see Ionization energies of the elements . All data from rutherfordium onwards is predicted.
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus as an example, the concise form is [Ne] 3s2 3p3. Here [Ne] refers to the core electrons which are the same as for the element neon (Ne), the last noble gas before phosphorus in the periodic table. The valence electrons are written explicitly for all atoms.
The Rydberg–Ritz combination principle is an empirical rule proposed by Walther Ritz in 1908 to describe the relationship of the spectral lines for all atoms, as a generalization of an earlier rule by Johannes Rydberg for the hydrogen atom and the alkali metals. The principle states that the spectral lines of any element include frequencies that are either the sum or the difference of the frequencies of two other lines. Lines of the spectra of elements could be predicted from existing lines. Since the frequency of light is proportional to the wavenumber or reciprocal wavelength, the principle can also be expressed in terms of wavenumbers which are the sum or difference of wavenumbers of two other lines.
Characterization, when used in materials science, refers to the broad and general process by which a material's structure and properties are probed and measured. It is a fundamental process in the field of materials science, without which no scientific understanding of engineering materials could be ascertained. The scope of the term often differs; some definitions limit the term's use to techniques which study the microscopic structure and properties of materials, while others use the term to refer to any materials analysis process including macroscopic techniques such as mechanical testing, thermal analysis and density calculation. The scale of the structures observed in materials characterization ranges from angstroms, such as in the imaging of individual atoms and chemical bonds, up to centimeters, such as in the imaging of coarse grain structures in metals.
This page deals with the electron affinity as a property of isolated atoms or molecules. Solid state electron affinities are not listed here.

An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions between such states they interact with a very specific frequency of electromagnetic radiation. This phenomenon serves as the basis for the International System of Units' (SI) definition of a second:
The second, symbol s, is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency, , the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom, to be 9192631770 when expressed in the unit Hz, which is equal to s−1.

Philip H. Bucksbaum is an American atomic physicist, the Marguerite Blake Wilbur Professor in Natural Science in the Departments of Physics, Applied Physics, and Photon Science at Stanford University and the SLAC National Accelerator Laboratory. He also directs the Stanford PULSE Institute.

William Clyde Martin Jr. was an American physicist. After receiving his Ph.D. degree from Princeton University in 1956, he joined the staff of the National Bureau of Standards, where he was employed until his retirement in 1998. As Chief of the NBS Atomic Spectroscopy Section from 1962 to 1998, he led the development of its reference data resources on the spectra of rare-earth elements, substantially increased its coverage of highly excited and ionized species, and pioneered the publication of NIST Standard Reference Data on the internet.
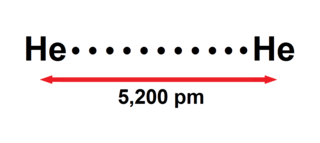
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.
{{cite journal}}: CS1 maint: location (link){{cite journal}}: CS1 maint: location (link){{cite journal}}: CS1 maint: location (link){{cite journal}}: CS1 maint: location (link){{cite journal}}: CS1 maint: bot: original URL status unknown (link) CS1 maint: location (link)As quoted at http://www.webelements.com/ from these sources: