
| Element | C1 | C2 | C3 | C4 | C5 | C6 | U1 | U2 |
|---|---|---|---|---|---|---|---|---|
| 01 H hydrogen | 1.40×10−3 | 1.520×10−3 | ||||||
| 02 He helium | 8×10−9 | |||||||
| 03 Li lithium | 2.0×10−5 | 2.0×10−5 | 1.8×10−5 | 1.3×10−5 | 1.37×10−5 | 2.0×10−5 | 2.2×10−5 | |
| 04 Be beryllium | 2.8×10−6 | 2.0×10−6 | 2×10−6 | 1.500×10−6 | 3.000×10−6 | |||
| 05 B boron | 1.0×10−5 | 7.0×10−6 | 9×10−6 | 1.0000×10−5 | 1.5000×10−5 | |||
| 06 C carbon | 2.00×10−4 | 1.80×10−4 | 3.76×10−3 | |||||
| 07 N nitrogen | 1.9×10−5 | 2.0×10−5 | 1.9×10−5 | |||||
| 08 O oxygen | 4.61×10−1 | 3.7×10−1 | 4.55000×10−1 | |||||
| 09 F fluorine | 5.85×10−4 | 4.6×10−4 | 5.44×10−4 | 5.25×10−4 | ||||
| 10 Ne neon | 5×10−9 | |||||||
| 11 Na sodium | 2.36×10−2 | 2.3×10−2 | 2.2700×10−2 | 2.3000×10−2 | 2.4400×10−2 | 3.1000×10−2 | 2.89×10−2 | 2.57×10−2 |
| 12 Mg magnesium | 2.33×10−2 | 2.8×10−2 | 2.7640×10−2 | 3.20×10−2 | 2.37×10−2 | 1.69×10−2 | 1.33×10−2 | 1.35×10−2 |
| 13 Al aluminium | 8.23×10−2 | 8.0×10−2 | 8.3000×10−2 | 8.4100×10−2 | 8.3050×10−2 | 8.5200×10−2 | 8.0400×10−2 | 7.7400×10−2 |
| 14 Si silicon | 2.82×10−1 | 2.7×10−1 | 2.72000×10−1 | 2.677×10−1 | 2.81×10−1 | 2.95×10−1 | 3.08×10−1 | 3.04×10−1 |
| 15 P phosphorus | 1.05×10−3 | 1.0×10−3 | 1.120×10−3 | 7.63×10−4 | 8.30×10−4 | |||
| 16 S sulfur | 3.50×10−4 | 3.0×10−4 | 3.40×10−4 | 8.81×10−4 | ||||
| 17 Cl chlorine | 1.45×10−4 | 1.9×10−4 | 1.26×10−4 | 1.900×10−3 | ||||
| 18 Ar argon | 3.5×10−6 | |||||||
| 19 K potassium | 2.09×10−2 | 1.7×10−2 | 1.8400×10−2 | 9.100×10−3 | 1.7600×10−2 | 1.7000×10−2 | 2.8000×10−2 | 2.5700×10−2 |
| 20 Ca calcium | 4.15×10−2 | 5.1×10−2 | 4.6600×10−2 | 5.2900×10−2 | 4.9200×10−2 | 3.4000×10−2 | 3.0000×10−2 | 2.9500×10−2 |
| 21 Sc scandium | 2.2×10−5 | 2.2×10−5 | 2.5×10−5 | 3.0×10−5 | 2.14×10−5 | 1.1×10−5 | 7×10−6 | |
| 22 Ti titanium | 5.65×10−3 | 8.6×10−3 | 6.320×10−3 | 5.400×10−3 | 5.250×10−3 | 3.600×10−3 | 3.000×10−3 | 3.120×10−3 |
| 23 V vanadium | 1.20×10−4 | 1.7×10−4 | 1.36×10−4 | 2.30×10−4 | 1.34×10−4 | 6.0×10−5 | 5.3×10−5 | |
| 24 Cr chromium | 1.02×10−4 | 9.6×10−5 | 1.22×10−4 | 1.85×10−4 | 1.46×10−4 | 5.6×10−5 | 3.5×10−5 | 3.5×10−5 |
| 25 Mn manganese | 9.50×10−4 | 1.0×10−3 | 1.060×10−3 | 1.400×10−3 | 8.47×10−4 | 1.000×10−3 | 6.00×10−4 | 5.27×10−4 |
| 26 Fe iron | 5.63×10−2 | 5.8×10−2 | 6.2000×10−2 | 7.07×10−2 | 4.92×10−2 | 3.8×10−2 | 3.50×10−2 | 3.09×10−2 |
| 27 Co cobalt | 2.5×10−5 | 2.8×10−5 | 2.9×10−5 | 2.9×10−5 | 2.54×10−5 | 1.0×10−5 | 1.2×10−5 | |
| 28 Ni nickel | 8.4×10−5 | 7.2×10−5 | 9.9×10−5 | 1.05×10−4 | 6.95×10−5 | 3.5×10−5 | 2×10−5 | 1.9×10−5 |
| 29 Cu copper | 6.0×10−5 | 5.8×10−5 | 6.8×10−5 | 7.5×10−5 | 4.7×10−5 | 2.5×10−5 | 1.4×10−5 | |
| 30 Zn zinc | 7.0×10−5 | 8.2×10−5 | 7.6×10−5 | 8.0×10−5 | 7.6×10−5 | 7.1×10−5 | 5.2×10−5 | |
| 31 Ga gallium | 1.9×10−5 | 1.7×10−5 | 1.9×10−5 | 1.8×10−5 | 1.86×10−5 | 1.7×10−5 | 1.4×10−5 | |
| 32 Ge germanium | 1.5×10−6 | 1.3×10−6 | 1.5×10−6 | 1.6×10−6 | 1.32×10−6 | 1.6×10−6 | ||
| 33 As arsenic | 1.8×10−6 | 2.0×10−6 | 1.8×10−6 | 1.0×10−6 | 2.03×10−6 | 1.5×10−6 | ||
| 34 Se selenium | 5×10−8 | 5×10−8 | 5×10−8 | 5×10−8 | 1.53×10−7 | 5×10−8 | ||
| 35 Br bromine | 2.4×10−6 | 4.0×10−6 | 2.5×10−6 | 6.95×10−6 | ||||
| 36 Kr krypton | 1×10−10 | |||||||
| 37 Rb rubidium | 9.0×10−5 | 7.0×10−5 | 7.8×10−5 | 3.2×10−5 | 7.90×10−5 | 6.1×10−5 | 1.12×10−4 | 1.10×10−4 |
| 38 Sr strontium | 3.70×10−4 | 4.5×10−4 | 3.84×10−4 | 2.60×10−4 | 2.93×10−4 | 5.03×10−4 | 3.50×10−4 | 3.16×10−4 |
| 39 Y yttrium | 3.3×10−5 | 3.5×10−7 | 3.1×10−5 | 2.0×10−5 | 1.4×10−5 | 2.2×10−5 | 2.1×10−5 | |
| 40 Zr zirconium | 1.65×10−4 | 1.4×10−4 | 1.62×10−4 | 1.00×10−4 | 2.10×10−4 | 1.90×10−4 | 2.40×10−4 | |
| 41 Nb niobium | 2.0×10−5 | 2.0×10−5 | 2.0×10−5 | 1.1000×10−5 | 1.3000×10−5 | 2.5000×10−5 | 2.6000×10−5 | |
| 42 Mo molybdenum | 1.2×10−6 | 1.2×10−6 | 1.2×10−6 | 1.000×10−6 | 1.500×10−6 | |||
| 43 Tc technetium | ||||||||
| 44 Ru ruthenium | 1×10−9 | 1×10−10 | ||||||
| 45 Rh rhodium | 1×10−9 | 1×10−10 | ||||||
| 46 Pd palladium | 1.5×10−8 | 3×10−9 | 1.5×10−8 | 1.0×10−9 | 5×10−10 | |||
| 47 Ag silver | 7.5×10−8 | 8×10−8 | 8×10−8 | 8.0×10−8 | 6.95×10−8 | 5.0×10−8 | ||
| 48 Cd cadmium | 1.5×10−7 | 1.8×10−7 | 1.6×10−7 | 9.8×10−8 | 1.00×10−7 | 9.8×10−8 | ||
| 49 In indium | 2.5×10−7 | 2×10−7 | 2.4×10−7 | 5.0×10−8 | 6.95×10−8 | 5.0×10−8 | ||
| 50 Sn tin | 2.3×10−6 | 1.5×10−6 | 2.1×10−6 | 2.500×10−6 | 5.500×10−6 | |||
| 51 Sb antimony | 2×10−7 | 2×10−7 | 2×10−7 | 2.00×10−7 | 2.03×10−7 | 2.00×10−7 | ||
| 52 Te tellurium | 1×10−9 | 1×10−9 | 2.03×10−9 | |||||
| 53 I iodine | 4.5×10−7 | 5×10−7 | 4.6×10−7 | 1.540×10−6 | ||||
| 54 Xe xenon | 3×10−11 | |||||||
| 55 Cs caesium | 3×10−6 | 1.6×10−6 | 2.6×10−6 | 1.000×10−6 | 1.310×10−6 | 3.700×10−6 | ||
| 56 Ba barium | 4.25×10−4 | 3.8×10−4 | 3.90×10−4 | 2.50000×10−4 | 5.42000×10−4 | 7.07000×10−4 | 5.50000×10−4 | 1.070000×10−3 |
| 57 La lanthanum | 3.9×10−5 | 5.0×10−5 | 3.5×10−5 | 1.6000×10−5 | 2.9000×10−5 | 2.8000×10−5 | 3.0000×10−5 | 3.200×10−6 |
| 58 Ce cerium | 6.65×10−5 | 8.3×10−5 | 6.6×10−5 | 3.3000×10−5 | 5.4200×10−5 | 5.7000×10−5 | 6.4000×10−5 | 6.5000×10−5 |
| 59 Pr praseodymium | 9.2×10−6 | 1.3×10−5 | 9.1×10−6 | 3.900×10−6 | 7.100×10−6 | |||
| 60 Nd neodymium | 4.15×10−5 | 4.4×10−5 | 4.0×10−5 | 1.6000×10−5 | 2.5400×10−5 | 2.3000×10−5 | 2.6000×10−5 | 2.6000×10−5 |
| 61 Pm promethium | ||||||||
| 62 Sm samarium | 7.05×10−6 | 7.7×10−6 | 7.0×10−6 | 3.500×10−6 | 5.590×10−6 | 4.100×10−6 | 4.500×10−6 | 4.500×10−6 |
| 63 Eu europium | 2.0×10−6 | 2.2×10−6 | 2.1×10−6 | 1.100×10−6 | 1.407×10−6 | 1.090×10−6 | 8.80×10−7 | 9.40×10−7 |
| 64 Gd gadolinium | 6.2×10−6 | 6.3×10−6 | 6.1×10−6 | 3.300×10−6 | 8.140×10−6 | 3.800×10−6 | 2.800×10−6 | |
| 65 Tb terbium | 1.2×10−6 | 1.0×10−6 | 1.2×10−6 | 6.00×10−7 | 1.020×10−6 | 5.30×10−7 | 6.40×10−7 | 4.80×10−7 |
| 66 Dy dysprosium | 5.2×10−6 | 8.5×10−6 | 3.700×10−6 | 6.102×10−6 | 3.500×10−6 | |||
| 67 Ho holmium | 1.3×10−6 | 1.6×10−6 | 1.3×10−6 | 7.80×10−7 | 1.860×10−6 | 8.00×10−7 | 6.20×10−7 | |
| 68 Er erbium | 3.5×10−6 | 3.6×10−6 | 3.5×10−6 | 2.200×10−6 | 3.390×10−6 | 2.300×10−6 | ||
| 69 Tm thulium | 5.2×10−7 | 5.2×10−7 | 5×10−7 | 3.20×10−7 | 2.40×10−7 | 3.30×10−7 | ||
| 70 Yb ytterbium | 3.2×10−6 | 3.4×10−6 | 3.1×10−6 | 2.200×10−6 | 3.390×10−6 | 1.530×10−6 | 2.200×10−6 | 1.500×10−6 |
| 71 Lu lutetium | 8×10−7 | 8×10−7 | 3.00×10−7 | 5.76×10−7 | 2.30×10−7 | 3.20×10−7 | 2.30×10−7 | |
| 72 Hf hafnium | 3.0×10−6 | 4×10−6 | 2.8×10−6 | 3.000×10−6 | 3.460×10−6 | 4.700×10−6 | 5.800×10−6 | 5.800×10−6 |
| 73 Ta tantalum | 2.0×10−6 | 2.4×10−6 | 1.7×10−6 | 1.000×10−6 | 2.203×10−6 | 2.200×10−6 | ||
| 74 W tungsten | 1.25×10−6 | 1.0×10−6 | 1.2×10−6 | 1.000×10−6 | 1.310×10−6 | 2.000×10−6 | ||
| 75 Re rhenium | 7×10−10 | 4×10−10 | 7×10−10 | 5×10−10 | 1.02×10−9 | 5×10−10 | ||
| 76 Os osmium | 1.5×10−9 | 2×10−10 | 5×10−9 | 1.02×10−9 | ||||
| 77 Ir iridium | 1×10−9 | 2×10−10 | 1×10−9 | 1×10−10 | 1.02×10−9 | 2×10−11 | ||
| 78 Pt platinum | 5×10−9 | 1×10−8 | ||||||
| 79 Au gold | 4×10−9 | 2×10−9 | 4×10−9 | 3.0×10−9 | 4.07×10−9 | 1.8×10−9 | ||
| 80 Hg mercury | 8.5×10−8 | 2×10−8 | 8×10−8 | |||||
| 81 Tl thallium | 8.5×10−7 | 4.7×10−7 | 7×10−7 | 3.60×10−7 | 7.50×10−7 | 5.20×10−7 | ||
| 82 Pb lead | 1.4×10−5 | 1.0×10−5 | 1.3×10−5 | 8.000×10−6 | 1.5000×10−5 | 2.0000×10−5 | 1.7000×10−5 | |
| 83 Bi bismuth | 8.5×10−9 | 4×10−9 | 8×10−9 | 6.0×10−8 | 1.27×10−7 | |||
| 84 Po polonium | 2×10−16 | |||||||
| 85 At astatine | ||||||||
| 86 Rn radon | 4×10−19 | |||||||
| 87 Fr francium | ||||||||
| 88 Ra radium | 9×10−13 | |||||||
| 89 Ac actinium | 5.5×10−16 | |||||||
| 90 Th thorium | 9.6×10−6 | 5.8×10−6 | 8.1×10−6 | 3.500×10−6 | 5.700×10−6 | 1.0700×10−5 | 1.0000×10−5 | |
| 91 Pa protactinium | 1.4×10−12 | |||||||
| 92 U uranium | 2.7×10−6 | 1.6×10−6 | 2.3×10−6 | 9.10×10−7 | 1.200×10−6 | 1.300×10−6 | 2.800×10−6 | 2.500×10−6 |
| 93 Np neptunium | ||||||||
| 94 Pu plutonium |
The established abundances of chemical elements in urban soils can be considered a geochemical (ecological and geochemical) characteristic, the accumulated impact of technogenic and natural processes at the beginning of the 21st century. The figures estimate average concentrations of chemical elements in the soils of more than 300 cities and settlements in Europe, Asia, Africa, Australia, and America. [1] Regardless of significant differences between abundances of several elements in urban soils and those values calculated for the Earth's crust, the element abundances in urban soils generally reflect those in the Earth's crust. With the development of technology the abundances may be refined.
Mass fraction, in mg/kg (ppm).
| Element | Atomic number | Abundance in urban soils |
|---|---|---|
| Ag | 47 | 0.37 |
| Al | 13 | 38200 |
| As | 33 | 15.9 |
| B | 5 | 45 |
| Ba | 56 | 853.12 |
| Be | 4 | 3.3 |
| Bi | 83 | 1.12 |
| C | 6 | 45100 |
| Ca | 20 | 53800 |
| Cd | 48 | 0.9 |
| Cl | 17 | 285 |
| Co | 27 | 14.1 |
| Cr | 24 | 80 |
| Cs | 55 | 5.0 |
| Cu | 29 | 39 |
| Fe | 26 | 22300 |
| Ga | 31 | 16.2 |
| Ge | 32 | 1.8 |
| H | 1 | 15000 |
| Hg | 80 | 0.88 |
| K | 19 | 13400 |
| La | 57 | 34 |
| Li | 3 | 49.5 |
| Mg | 12 | 7900 |
| Mn | 25 | 729 |
| Mo | 42 | 2.4 |
| N | 7 | 10000 |
| Na | 11 | 5800 |
| Nb | 41 | 15.7 |
| Ni | 28 | 33 |
| O | 8 | 490000 |
| P | 15 | 1200 |
| Pb | 82 | 54.5 |
| Rb | 37 | 58 |
| S | 16 | 1200 |
| Sb | 51 | 1.0 |
| Sc | 21 | 9.4 |
| Si | 14 | 289000 |
| Sn | 50 | 6.8 |
| Sr | 38 | 458 |
| Ta | 73 | 1.5 |
| Ti | 22 | 4758 |
| Tl | 81 | 1.1 |
| V | 23 | 104.9 |
| W | 74 | 2.9 |
| Y | 39 | 23.4 |
| Yb | 70 | 2.4 |
| Zn | 30 | 158 |
| Zr | 40 | 255.6 |
Mass concentration, in kg/L. (The average density of sea water in the surface is 1.025 kg/L)
| Element | W1 | W2 |
|---|---|---|
| 01 H hydrogen | 1.08×10−1 | 1.1×10−1 |
| 02 He helium | 7×10−12 | 7.2×10−12 |
| 03 Li lithium | 1.8×10−7 | 1.7×10−7 |
| 04 Be beryllium | 5.6×10−12 | 6×10−13 |
| 05 B boron | 4.44×10−6 | 4.4×10−6 |
| 06 C carbon | 2.8×10−5 | 2.8×10−5 |
| 07 N nitrogen | 5×10−7 | 1.6×10−5 |
| 08 O oxygen | 8.57×10−1 | 8.8×10−1 |
| 09 F fluorine | 1.3×10−6 | 1.3×10−6 |
| 10 Ne neon | 1.2×10−10 | 1.2×10−10 |
| 11 Na sodium | 1.08×10−2 | 1.1×10−2 |
| 12 Mg magnesium | 1.29×10−3 | 1.3×10−3 |
| 13 Al aluminium | 2×10−9 | 1×10−9 |
| 14 Si silicon | 2.2×10−6 | 2.9×10−6 |
| 15 P phosphorus | 6×10−8 | 8.8×10−8 |
| 16 S sulfur | 9.05×10−4 | 9.0×10−4 |
| 17 Cl chlorine | 1.94×10−2 | 1.9×10−2 |
| 18 Ar argon | 4.5×10−7 | 4.5×10−7 |
| 19 K potassium | 3.99×10−4 | 3.9×10−4 |
| 20 Ca calcium | 4.12×10−4 | 4.1×10−4 |
| 21 Sc scandium | 6×10−13 | < 4×10−12 |
| 22 Ti titanium | 1×10−9 | 1×10−9 |
| 23 V vanadium | 2.5×10−9 | 1.9×10−9 |
| 24 Cr chromium | 3×10−10 | 2×10−10 |
| 25 Mn manganese | 2×10−10 | 1.9×10−9 |
| 26 Fe iron | 2×10−9 | 3.4×10−9 |
| 27 Co cobalt | 2×10−11 | 3.9×10−10 |
| 28 Ni nickel | 5.6×10−10 | 6.6×10−9 |
| 29 Cu copper | 2.5×10−10 | 2.3×10−8 |
| 30 Zn zinc | 4.9×10−9 | 1.1×10−8 |
| 31 Ga gallium | 3×10−11 | 3×10−11 |
| 32 Ge germanium | 5×10−11 | 6×10−11 |
| 33 As arsenic | 3.7×10−9 | 2.6×10−9 |
| 34 Se selenium | 2×10−10 | 9.0×10−11 |
| 35 Br bromine | 6.73×10−5 | 6.7×10−5 |
| 36 Kr krypton | 2.1×10−10 | 2.1×10−10 |
| 37 Rb rubidium | 1.2×10−7 | 1.2×10−7 |
| 38 Sr strontium | 7.9×10−6 | 8.1×10−6 |
| 39 Y yttrium | 1.3×10−11 | 1.3×10−12 |
| 40 Zr zirconium | 3×10−11 | 2.6×10−11 |
| 41 Nb niobium | 1×10−11 | 1.5×10−11 |
| 42 Mo molybdenum | 1×10−8 | 1.0×10−8 |
| 43 Tc technetium | ||
| 44 Ru ruthenium | 7×10−13 | |
| 45 Rh rhodium | ||
| 46 Pd palladium | ||
| 47 Ag silver | 4×10−11 | 2.8×10−10 |
| 48 Cd cadmium | 1.1×10−10 | 1.1×10−10 |
| 49 In indium | 2×10−8 | |
| 50 Sn tin | 4×10−12 | 8.1×10−10 |
| 51 Sb antimony | 2.4×10−10 | 3.3×10−10 |
| 52 Te tellurium | ||
| 53 I iodine | 6×10−8 | 6.4×10−8 |
| 54 Xe xenon | 5×10−11 | 4.7×10−11 |
| 55 Cs caesium | 3×10−10 | 3.0×10−10 |
| 56 Ba barium | 1.3×10−8 | 2.1×10−8 |
| 57 La lanthanum | 3.4×10−12 | 3.4×10−12 |
| 58 Ce cerium | 1.2×10−12 | 1.2×10−12 |
| 59 Pr praseodymium | 6.4×10−13 | 6.4×10−13 |
| 60 Nd neodymium | 2.8×10−12 | 2.8×10−12 |
| 61 Pm promethium | ||
| 62 Sm samarium | 4.5×10−13 | 4.5×10−13 |
| 63 Eu europium | 1.3×10−13 | 1.3×10−13 |
| 64 Gd gadolinium | 7×10−13 | 7.0×10−13 |
| 65 Tb terbium | 1.4×10−13 | 1.4×10−12 |
| 66 Dy dysprosium | 9.1×10−13 | 9.1×10−13 |
| 67 Ho holmium | 2.2×10−13 | 2.2×10−13 |
| 68 Er erbium | 8.7×10−13 | 8.7×10−12 |
| 69 Tm thulium | 1.7×10−13 | 1.7×10−13 |
| 70 Yb ytterbium | 8.2×10−13 | 8.2×10−13 |
| 71 Lu lutetium | 1.5×10−13 | 1.5×10−13 |
| 72 Hf hafnium | 7×10−12 | < 8×10−12 |
| 73 Ta tantalum | 2×10−12 | < 2.5×10−12 |
| 74 W tungsten | 1×10−10 | < 1×10−12 |
| 75 Re rhenium | 4×10−12 | |
| 76 Os osmium | ||
| 77 Ir iridium | ||
| 78 Pt platinum | ||
| 79 Au gold | 4×10−12 | 1.1×10−11 |
| 80 Hg mercury | 3×10−11 | 1.5×10−10 |
| 81 Tl thallium | 1.9×10−11 | |
| 82 Pb lead | 3×10−11 | 3×10−11 |
| 83 Bi bismuth | 2×10−11 | 2×10−11 |
| 84 Po polonium | 1.5×10−20 | |
| 85 At astatine | ||
| 86 Rn radon | 6×10−22 | |
| 87 Fr francium | ||
| 88 Ra radium | 8.9×10−17 | |
| 89 Ac actinium | ||
| 90 Th thorium | 1×10−12 | 1.5×10−12 |
| 91 Pa protactinium | 5×10−17 | |
| 92 U uranium | 3.2×10−9 | 3.3×10−9 |
| 93 Np neptunium | ||
| 94 Pu plutonium |
Atom mole fraction relative to silicon = 1.
| Element | S1 | Y1 | Y2 |
|---|---|---|---|
| 01 H hydrogen | 2.8×104 | 2.8×104* | 2.79×104 |
| 02 He helium | 2.7×103 | 2.7×103* | 2.72×103 |
| 03 Li lithium | 4.0×10−7 | 5.7×10−5 | 5.71×10−5 (9.2%) |
| 04 Be beryllium | 4.0×10−7 | 7.0×10−7 | 7.30×10−7 (9.5%) |
| 05 B boron | 1.1×10−5 | 2.1×10−5 | 2.12×10−5 (10%) |
| 06 C carbon | 1.0×101 | 1.0×101* | 1.01×101 |
| 07 N nitrogen | 3.1×100 | 3.1×100* | 3.13×100 |
| 08 O oxygen | 2.4×101 | 2.4×101* | 2.38×101 (10%) |
| 09 F fluorine | about 1.0×10−3 | 8.5×10−4 | 8.43×10−4 (15%) |
| 10 Ne neon | 3.0×100 | 3.0×100* | 3.44×100 (14%) |
| 11 Na sodium | 6.0×10−2 | 5.7×10−2 | 5.74×10−2 (7.1%) |
| 12 Mg magnesium | 1.0×100 | 1.1×100 | 1.074×100 (3.8%) |
| 13 Al aluminium | 8.3×10−2 | 8.5×10−2 | 8.49×10−2 (3.6%) |
| 14 Si silicon | 1.0×100 | 1.0×100 | 1.0×100 (4.4%) |
| 15 P phosphorus | 8.0×10−3 | 1.0×10−2 | 1.04×10−2 (10%) |
| 16 S sulfur | 4.5×10−1 | 5.2×10−1 | 5.15×10−1 (13%) |
| 17 Cl chlorine | about 9.0×10−3 | 5.2×10−3 | 5.24×10−3 (15%) |
| 18 Ar argon | 1.0×10−1* | 1.0×10−1* | 1.01×10−1 (6%) |
| 19 K potassium | 3.7×10−3 | 3.8×10−3 | 3.77×10−3 (7.7%) |
| 20 Ca calcium | 6.4×10−2 | 6.1×10−2 | 6.11×10−2 (7.1%) |
| 21 Sc scandium | 3.5×10−5 | 3.4×10−5 | 3.42×10−5 (8.6%) |
| 22 Ti titanium | 2.7×10−3 | 2.4×10−3 | 2.40×10−3 (5.0%) |
| 23 V vanadium | 2.8×10−4 | 2.9×10−4 | 2.93×10−4 (5.1%) |
| 24 Cr chromium | 1.3×10−2 | 1.3×10−2 | 1.35×10−2 (7.6%) |
| 25 Mn manganese | 6.9×10−3 | 9.5×10−3 | 9.55×10−3 (9.6%) |
| 26 Fe iron | 9.0×10−1 | 9.0×10−1 | 9.00×10−1 (2.7%) |
| 27 Co cobalt | 2.3×10−3 | 2.3×10−3 | 2.25×10−3 (6.6%) |
| 28 Ni nickel | 5.0×10−2 | 5.0×10−2 | 4.93×10−2 (5.1%) |
| 29 Cu copper | 4.5×10−4 | 5.2×10−4 | 5.22×10−4 (11%) |
| 30 Zn zinc | 1.1×10−3 | 1.3×10−3 | 1.26×10−3 (4.4%) |
| 31 Ga gallium | 2.1×10−5 | 3.8×10−5 | 3.78×10−5 (6.9%) |
| 32 Ge germanium | 7.2×10−5 | 1.2×10−4 | 1.19×10−4 (9.6%) |
| 33 As arsenic | 6.6×10−6 | 6.56×10−6 (12%) | |
| 34 Se selenium | 6.3×10−5 | 6.21×10−5 (6.4%) | |
| 35 Br bromine | 1.2×10−5 | 1.18×10−5 (19%) | |
| 36 Kr krypton | 4.8×10−5 | 4.50×10−5 (18%) | |
| 37 Rb rubidium | 1.1×10−5 | 7.0×10−6 | 7.09×10−6 (6.6%) |
| 38 Sr strontium | 2.2×10−5 | 2.4×10−5 | 2.35×10−5 (8.1%) |
| 39 Y yttrium | 4.9×10−6 | 4.6×10−6 | 4.64×10−6 (6.0%) |
| 40 Zr zirconium | 1.12×10−5 | 1.14×10−5 | 1.14×10−5 (6.4%) |
| 41 Nb niobium | 7.0×10−7 | 7.0×10−7 | 6.98×10−7 (1.4%) |
| 42 Mo molybdenum | 2.3×10−6 | 2.6×10−6 | 2.55×10−6 (5.5%) |
| 43 Tc technetium | |||
| 44 Ru ruthenium | 1.9×10−6 | 1.9×10−6 | 1.86×10−6 (5.4%) |
| 45 Rh rhodium | 4.0×10−7 | 3.4×10−7 | 3.44×10−7 (8%) |
| 46 Pd palladium | 1.4×10−6 | 1.4×10−6 | 1.39×10−6 (6.6%) |
| 47 Ag silver | about 2.0×10−7 | 4.9×10−7 | 4.86×10−7 (2.9%) |
| 48 Cd cadmium | 2.0×10−6 | 1.6×10−6 | 1.61×10−6 (6.5%) |
| 49 In indium | about 1.3×10−6 | 1.9×10−7 | 1.84×10−7 (6.4%) |
| 50 Sn tin | about 3.0×10−6 | 3.9×10−6 | 3.82×10−6 (9.4%) |
| 51 Sb antimony | about 3.0×10−7 | 3.1×10−7 | 3.09×10−7 (18%) |
| 52 Te tellurium | 4.9×10−6 | 4.81×10−6 (10%) | |
| 53 I iodine | 9.0×10−7 | 9.00×10−7 (21%) | |
| 54 Xe xenon | 4.8×10−6 | 4.70×10−6 (20%) | |
| 55 Cs caesium | 3.7×10−7 | 3.72×10−7 (5.6%) | |
| 56 Ba barium | 3.8×10−6 | 4.5×10−6 | 4.49×10−6 (6.3%) |
| 57 La lanthanum | 5.0×10−7 | 4.4×10−7 | 4.46×10−7 (2.0%) |
| 58 Ce cerium | 1.0×10−6 | 1.1×10−6 | 1.136×10−6 (1.7%) |
| 59 Pr praseodymium | 1.4×10−7 | 1.7×10−7 | 1.669×10−7 (2.4%) |
| 60 Nd neodymium | 9.0×10−7 | 8.3×10−7 | 8.279×10−7 (1.3%) |
| 61 Pm promethium | |||
| 62 Sm samarium | 3.0×10−7 | 2.6×10−7 | 2.582×10−7 (1.3%) |
| 63 Eu europium | 9.0×10−8 | 9.7×10−8 | 9.73×10−8 (1.6%) |
| 64 Gd gadolinium | 3.7×10−7 | 3.3×10−7 | 3.30×10−7 (1.4%) |
| 65 Tb terbium | about 2.0×10−8 | 6.0×10−8 | 6.03×10−8 (2.2%) |
| 66 Dy dysprosium | 3.5×10−7 | 4.0×10−7 | 3.942×10−7 (1.4%) |
| 67 Ho holmium | about 5.0×10−8 | 8.9×10−8 | 8.89×10−8 (2.4%) |
| 68 Er erbium | 2.4×10−7 | 2.5×10−7 | 2.508×10−7 (1.3%) |
| 69 Tm thulium | about 3.0×10−8 | 3.8×10−8 | 3.78×10−8 (2.3%) |
| 70 Yb ytterbium | 3.4×10−7 | 2.5×10−7 | 2.479×10−7 (1.6%) |
| 71 Lu lutetium | about 1.5×10−7 | 3.7×10−8 | 3.67×10−8 (1.3%) |
| 72 Hf hafnium | 2.1×10−7 | 1.5×10−7 | 1.54×10−7 (1.9%) |
| 73 Ta tantalum | 3.8×10−8 | 2.07×10−8 (1.8%) | |
| 74 W tungsten | about 3.6×10−7 | 1.3×10−7 | 1.33×10−7 (5.1%) |
| 75 Re rhenium | 5.0×10−8 | 5.17×10−8 (9.4%) | |
| 76 Os osmium | 8.0×10−7 | 6.7×10−7 | 6.75×10−7 (6.3%) |
| 77 Ir iridium | 6.0×10−7 | 6.6×10−7 | 6.61×10−7 (6.1%) |
| 78 Pt platinum | about 1.8×10−6 | 1.34×10−6 | 1.34×10−6 (7.4%) |
| 79 Au gold | about 3.0×10−7 | 1.9×10−7 | 1.87×10−7 (15%) |
| 80 Hg mercury | 3.4×10−7 | 3.40×10−7 (12%) | |
| 81 Tl thallium | about 2.0×10−7 | 1.9×10−7 | 1.84×10−7 (9.4%) |
| 82 Pb lead | 2.0×10−6 | 3.1×10−6 | 3.15×10−6 (7.8%) |
| 83 Bi bismuth | 1.4×10−7 | 1.44×10−7 (8.2%) | |
| 84 Po polonium | |||
| 85 At astatine | |||
| 86 Rn radon | |||
| 87 Fr francium | |||
| 88 Ra radium | |||
| 89 Ac actinium | |||
| 90 Th thorium | 5.0×10−8 | 4.5×10−8 | 3.35×10−8 (5.7%) |
| 91 Pa protactinium | |||
| 92 U uranium | 1.8×10−8 | 9.00×10−9 (8.4%) | |
| 93 Np neptunium | |||
| 94 Pu plutonium |
Due to the estimate nature of these values, no single recommendations are given. All values are normalized for these tables. Underlined zeroes indicate figures of indeterminable significance that were present in the source notation.
Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the entire Solar System, and has made important contributions to the understanding of a number of processes including mantle convection, the formation of planets and the origins of granite and basalt. It is an integrated field of chemistry and geology.
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass of these isotopes is the atomic weight listed for the element in the periodic table. The abundance of an isotope varies from planet to planet, and even from place to place on the Earth, but remains relatively constant in time.
The Goldschmidt classification, developed by Victor Goldschmidt (1888–1947), is a geochemical classification which groups the chemical elements within the Earth according to their preferred host phases into lithophile (rock-loving), siderophile (iron-loving), chalcophile, and atmophile (gas-loving) or volatile.
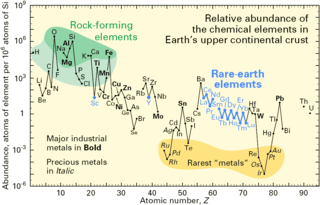
The abundance of the chemical elements is a measure of the occurrences of the chemical elements relative to all other elements in a given environment. Abundance is measured in one of three ways: by mass fraction, by mole fraction, or by volume fraction. Volume fraction is a common abundance measure in mixed gases such as planetary atmospheres, and is similar in value to molecular mole fraction for gas mixtures at relatively low densities and pressures, and ideal gas mixtures. Most abundance values in this article are given as mass fractions.
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope-ratio mass spectrometry, and can reveal information about the ages and origins of rock, air or water bodies, or processes of mixing between them.
Earth's crust is its thick outer shell of rock, referring to less than one percent of the planet's radius and volume. It is the top component of the lithosphere, a solidified division of Earth's layers that includes the crust and the upper part of the mantle. The lithosphere is broken into tectonic plates whose motion allows heat to escape the interior of Earth into space.
Elastic properties describe the reversible deformation of a material to an applied stress. They are a subset of the material properties that provide a quantitative description of the characteristics of a material, like its strength.
The abundance of elements in Earth's crust is shown in tabulated form with the estimated crustal abundance for each chemical element shown as mg/kg, or parts per million (ppm) by mass.
The Petrological Database of the Ocean Floor (PetDB) is a relational database for global geochemical data on igneous and metamorphic rocks generated at mid-ocean ridges including back-arc basins, young seamounts, and old oceanic crust, as well as ophiolites and terrestrial xenoliths from the mantle and lower crust and diamond geochemistry. These data are obtained by analyses of whole rock powders, volcanic glasses, and minerals by a wide range of techniques including mass spectrometry, atomic emission spectrometry, x-ray fluorescence spectrometry, and wet chemical analyses. Data are compiled from the scientific literature by PetDB data managers, and entered after methodical metadata review. Members of the scientific community can also suggest entry of specific data that has been entered into the EarthChem Library. PetDB is administered by the EarthChem group under the IEDA facility at LDEO headed by K. Lehnert. PetDB is supported by the U.S. National Science Foundation.
The following outline is provided as an overview of and topical guide to geology:

Extraterrestrial material refers to natural objects now on Earth that originated in outer space. Such materials include cosmic dust and meteorites, as well as samples brought to Earth by sample return missions from the Moon, asteroids and comets, as well as solar wind particles.
CI chondrites, also called C1 chondrites or Ivuna-type carbonaceous chondrites, are a group of rare carbonaceous chondrite, a type of stony meteorite. They are named after the Ivuna meteorite, the type specimen. CI chondrites have been recovered in France, Canada, India, and Tanzania. Their overall chemical composition closely resembles the elemental composition of the Sun, more so than any other type of meteorite.
Potassium–calcium dating, abbreviated K–Ca dating, is a radiometric dating method used in geochronology. It is based upon measuring the ratio of a parent isotope of potassium to a daughter isotope of calcium. This form of radioactive decay is accomplished through beta decay.

Hadean zircon is the oldest-surviving crustal material from the Earth's earliest geological time period, the Hadean eon, about 4 billion years ago. Zircon is a mineral that is commonly used for radiometric dating because it is highly resistant to chemical changes and appears in the form of small crystals or grains in most igneous and metamorphic host rocks.
The K/U Ratio is the ratio of a slightly volatile element, potassium (K), to a highly refractory element, uranium (U). It is a useful way to measure the presence of volatile elements on planetary surfaces. The K/U ratio helps explain the evolution of the planetary system and the origin of Earth's moon.

The fluorine cycle is the series of biogeochemical processes through which fluorine moves through the lithosphere, hydrosphere, atmosphere, and biosphere. Fluorine originates from the Earth’s crust, and its cycling between various sources and sinks is modulated by a variety of natural and anthropogenic processes.
From these sources in an online version of David R. Lide (ed.), CRC Handbook of Chemistry and Physics, 85th Edition. CRC Press. Boca Raton, Florida (2005). Section 14, Geophysics, Astronomy, and Acoustics; Abundance of Elements in the Earth's Crust and in the Sea:
National Physical Laboratory, Kaye and Laby Tables of Physical & Chemical Constants (2005). Section 3.1.3, Abundances of the elements, B.E.J. Pagel
A. Earnshaw, N. Greenwood, Chemistry of the Elements, 2nd edition, Butterworth-Heinemann, (1997). ISBN 0-7506-3365-4 Appendix 4, Abundance of Elements in Crustal Rocks.
Newsom, Horton E. (1995), "Composition of the Solar System, Planets, Meteorites, and Major Terrestrial Reservoirs", in Ahrens, Thomas J. (ed.), Global Earth Physics : A Handbook of Physical Constants, AGU Reference Shelf, vol. 1, American Geophysical Union, Tables 1, 14, 15., Bibcode:1995geph.conf.....A, doi:10.1029/RF001, ISBN 0-87590-851-9