Related Research Articles

Severe acute respiratory syndrome (SARS) is a viral respiratory disease of zoonotic origin caused by the virus SARS-CoV-1, the first identified strain of the SARS-related coronavirus. The first known cases occurred in November 2002, and the syndrome caused the 2002–2004 SARS outbreak. In the 2010s, Chinese scientists traced the virus through the intermediary of Asian palm civets to cave-dwelling horseshoe bats in Xiyang Yi Ethnic Township, Yunnan.

Sir Jeremy James Farrar is a British medical researcher who serves as Chief Scientist at the World Health Organization since 2023. He was previously the director of The Wellcome Trust from 2013 to 2023 and a professor of tropical medicine at the University of Oxford.
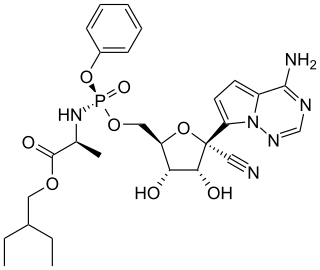
Remdesivir, sold under the brand name Veklury, is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is administered via injection into a vein. During the COVID‑19 pandemic, remdesivir was approved or authorized for emergency use to treat COVID‑19 in numerous countries.

Coronavirus disease 2019 (COVID-19) is a contagious disease caused by the virus SARS-CoV-2. The first known case was identified in Wuhan, China, in December 2019. The disease quickly spread worldwide, resulting in the COVID-19 pandemic.

Dame Sarah Catherine Gilbert FRS is an English vaccinologist who is a Professor of Vaccinology at the University of Oxford and co-founder of Vaccitech. She specialises in the development of vaccines against influenza and emerging viral pathogens. She led the development and testing of the universal flu vaccine, which underwent clinical trials in 2011.
Trudie Lang is a Professor of Global Health Research at the University of Oxford. She specialises in clinical trials research capacity building in low-resource setting, and helped to organise the trial for the drug brincidofovir during the 2014 Ebola virus outbreak.

Christian Heinrich Maria Drosten is a German virologist whose research focus is on novel viruses (emergent viruses). During the COVID-19 pandemic, Drosten came to national prominence as an expert on the implications and actions required to combat the illness in Germany.
The New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) is an advisory body that advises the United Kingdom Government's Chief Medical Advisor / Chief Medical Officer for England, who in turn advises the UK Department of Health and Social Care and relevant ministers regarding threats from viral respiratory tract infections. The body replaced the UK Scientific Pandemic Influenza Advisory Committee (SPI) as part of a move to expand the scope to cover the threat of other respiratory viruses, besides pandemic influenza. The inaugural meeting was held on 19 December 2014 where the terms of reference were agreed. The group has been advising the Department of Health for some years and minutes of meetings are now regularly published, backdated to 2014. As of 2020, the group has been advising specifically on the COVID-19 pandemic.

Drug repositioning is the repurposing of an approved drug for the treatment of a different disease or medical condition than that for which it was originally developed. This is one line of scientific research which is being pursued to develop safe and effective COVID-19 treatments. Other research directions include the development of a COVID-19 vaccine and convalescent plasma transfusion.

COVID-19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus disease 2019 (COVID-19). From early 2020 through 2021, several hundred drug companies, biotechnology firms, university research groups, and health organizations were developing therapeutic candidates for COVID-19 disease in various stages of preclinical or clinical research, with 419 potential COVID-19 drugs in clinical trials, as of April 2021.

Convalescent plasma is the blood plasma collected from a survivor of an infectious disease. This plasma contains antibodies specific to a pathogen and can be used therapeutically by providing passive immunity when transfusing it to a newly infected patient with the same condition. Convalescent plasma can be transfused as it has been collected or become the source material for the hyperimmune serum which consists largely of IgG but also includes IgA and IgM. or as source material for anti-pathogen monoclonal antibodies, Collection is typically achieved by apheresis, but in low-to-middle income countries, the treatment can be administered as convalescent whole blood.

Müge Çevik is a physician who is an infectious diseases researcher and science communicator at the University of St Andrews. Her research considers HIV, viral hepatitis, emerging infections and tropical infections in developing countries. During the COVID-19 pandemic, Çevik was an advisor to the Chief Medical Officer of Scotland and the World Health Organization, and is a member of New and Emerging Respiratory Virus Threats Advisory Group - an expert committee of the UK Department of Health advising Scientific Advisory Group for Emergencies.
Although several medications have been approved in different countries as of April 2022, not all countries have these medications. Patients with mild to moderate symptoms who are in the risk groups can take nirmatrelvir/ritonavir or remdesivir, either of which reduces the risk of serious illness or hospitalization. In the US, the Biden Administration COVID-19 action plan includes the Test to Treat initiative, where people can go to a pharmacy, take a COVID test, and immediately receive free Paxlovid if they test positive.

The Randomised Evaluation of COVID-19 Therapy is a large-enrollment clinical trial of possible treatments for people in the United Kingdom admitted to hospital with severe COVID-19 infection. The trial was later expanded to Indonesia, Nepal and Vietnam. The trial has tested ten interventions on adults: eight repurposed drugs, one newly developed drug and convalescent plasma.
Donna Elizabeth Davies is a British biochemist and professor of respiratory cell and molecular biology at the University of Southampton. In 2003, Davies was the co-founder of Synairgen, an interferon-beta drug designed to treat patients with asthma and chronic obstructive pulmonary disease.
Synairgen is a University spin-off and public limited company (plc) working in drug discovery and biotechnology. It was founded in 2003 by University of Southampton professors Stephen Holgate, Donna E. Davies and Ratko Djukanovic. The company is developing an inhaled formulation of interferon-beta for severe viral respiratory diseases including COVID-19.
Sir Martin Jonathan Landray is a British physician, epidemiologist and data scientist who serves as a Professor of Medicine & Epidemiology at the University of Oxford. Landray designs, conducts and analyses large-scale randomised control trials; including practice-changing international trials that have recruited over 100,000 individuals. Landray previously led the health informatics team that enabled the collection and management of data for the UK Biobank on over half a million people.

Shabir Ahmed Madhi is a South African physician who is professor of vaccinology and director of the South African Medical Research Council Respiratory and Meningeal Pathogens Research Unit at the University of the Witwatersrand, and National Research Foundation/Department of Science and Technology Research Chair in Vaccine Preventable Diseases. In January 2021, he was appointed Dean of the Faculty of Health Sciences at the University of the Witwateratand.

Chloroquine and hydroxychloroquine are anti-malarial medications also used against some auto-immune diseases. Chloroquine, along with hydroxychloroquine, was an early experimental treatment for COVID-19. Neither drug prevents SARS-CoV-2 infection.
The International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) is an international research initiative based in Oxford, England. It is hosted at the Nuffield Department of Medicine within the University of Oxford and led by the Epidemic diseases Research Group Oxford (ERGO). ISARIC is funded by the Bill & Melinda Gates Foundation, Foreign, Commonwealth and Development Office, and Wellcome Trust.
References
- ↑ "ISRCTN - ISRCTN50189673: A randomised trial of treatments to prevent death in patients hospitalised with COVID-19 (coronavirus)". www.isrctn.com. Retrieved 6 September 2020.
- ↑ "Large-scale trial for coronavirus drugs launches in UK". Clinical Trials Arena. 6 April 2020. Retrieved 7 September 2020.
- ↑ "Managing clinical trials during the pandemic — Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences". www.ndorms.ox.ac.uk. Retrieved 7 September 2020.
- ↑ "Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19 | University of Oxford". www.ox.ac.uk. Retrieved 6 September 2020.
- ↑ "Prime Minister's statement on coronavirus (COVID-19): 16 June 2020". GOV.UK. 16 June 2020. Retrieved 6 September 2020.
- ↑ Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. (July 2020). "Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report". The New England Journal of Medicine. 384 (8): 693–704. doi:10.1056/NEJMoa2021436. PMC 7383595 . PMID 32678530.
- 1 2 Sample, Ian (24 April 2020). "Who's who on secret scientific group advising UK government?". The Guardian. Retrieved 24 April 2020.
- ↑ "New and Emerging Respiratory Virus Threats Advisory Group". gov.uk. Retrieved 24 April 2020.
- ↑ "No. 63377". The London Gazette (Supplement). 12 June 2021. p. B2.