Related Research Articles
A polyphosphate is a salt or ester of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic (also called, ring) structures. In biology, the polyphosphate esters ADP and ATP are involved in energy storage. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 ppm. GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis, and carbohydrate metabolism, respectively. Polyphosphates are also used as food additives, marked E452.

Pseudomonas is a genus of Gram-negative bacteria belonging to the family Pseudomonadaceae in the class Gammaproteobacteria. The 313 members of the genus demonstrate a great deal of metabolic diversity and consequently are able to colonize a wide range of niches. Their ease of culture in vitro and availability of an increasing number of Pseudomonas strain genome sequences has made the genus an excellent focus for scientific research; the best studied species include P. aeruginosa in its role as an opportunistic human pathogen, the plant pathogen P. syringae, the soil bacterium P. putida, and the plant growth-promoting P. fluorescens, P. lini, P. migulae, and P. graminis.

Porins are beta barrel proteins that cross a cellular membrane and act as a pore, through which molecules can diffuse. Unlike other membrane transport proteins, porins are large enough to allow passive diffusion, i.e., they act as channels that are specific to different types of molecules. They are present in the outer membrane of gram-negative bacteria and some gram-positive mycobacteria, the outer membrane of mitochondria, and the outer chloroplast membrane.
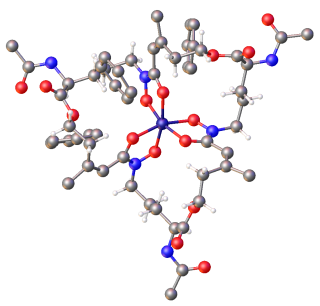
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is now being appreciated, siderophores are among the strongest (highest affinity) Fe3+ binding agents known. Phytosiderophores are siderophores produced by plants.

The Pseudomonadaceae are a family of bacteria which includes the genera Azomonas, Azorhizophilus, Azotobacter, Mesophilobacter, Pseudomonas, and Rugamonas. The family Azotobacteraceae was recently reclassified into this family.

Pseudomonas aeruginosa is a common encapsulated, Gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, P. aeruginosa is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes. P. aeruginosa is able to selectively inhibit various antibiotics from penetrating its outer membrane - and has high resistance to several antibiotics. According to the World Health Organization P. aeruginosa poses one of the greatest threats to humans in terms of antibiotic resistance.

Carbapenems are a class of very effective antibiotic agents most commonly used for treatment of severe bacterial infections. This class of antibiotics is usually reserved for known or suspected multidrug-resistant (MDR) bacterial infections. Similar to penicillins and cephalosporins, carbapenems are members of the beta-lactam antibiotics drug class, which kill bacteria by binding to penicillin-binding proteins, thus inhibiting bacterial cell wall synthesis. However, these agents individually exhibit a broader spectrum of activity compared to most cephalosporins and penicillins. Furthermore, carbapenems are typically unaffected by emerging antibiotic resistance, even to other beta-lactams.
The Transporter Classification Database is an International Union of Biochemistry and Molecular Biology (IUBMB)-approved classification system for membrane transport proteins, including ion channels.

Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of enzymes called transpeptidases. Specifically, PBPs are DD-transpeptidases.
4-amino-4-deoxychorismate lyase is an enzyme that participates in folate biosynthesis by catalyzing the production of PABA by the following reaction
In enzymology, a 2-aminoethylphosphonate—pyruvate transaminase is an enzyme that catalyzes the chemical reaction

Multiple inositol polyphosphate phosphatase 1 is an enzyme that in humans is encoded by the MINPP1 gene.

Gallium maltolate is a coordination complex consisting of a trivalent gallium cation coordinated to three maltolate ligands. The compound is a potential therapeutic agent for cancer, infectious disease, and inflammatory disease. A cosmetic skin cream containing gallium maltolate is marketed under the name Gallixa. It is a colorless solid with significant solubility in both water and lipids.
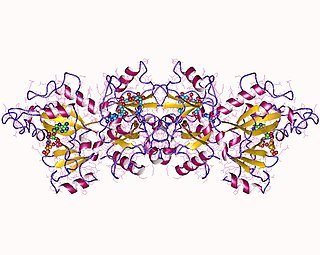
The Pseudomonas exotoxin is an exotoxin produced by Pseudomonas aeruginosa. Vibrio cholerae produces a similar protein called the Cholix toxin.

The Pseudomon-1 RNA motif is a conserved RNA identified by bioinformatics. It is used by most species whose genomes have been sequenced and that are classified within the genus Pseudomonas, and is also present in Azotobacter vinelandii, a closely related species. It is presumed to function as a non-coding RNA. Pseudomon-1 RNAs consistently have a downstream rho-independent transcription terminator.
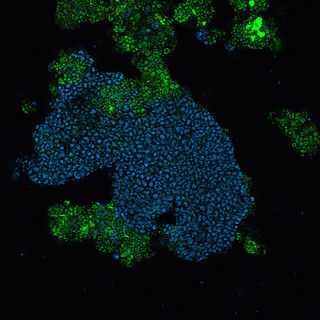
Candidatus Accumulibacter phosphatis (CAP) is an unclassified type of Betaproteobacteria that is a common bacterial community member of sewage treatment and wastewater treatment plants performing enhanced biological phosphorus removal (EBPR) and is a polyphosphate-accumulating organism. The role of CAP in EBPR was elucidated using culture-independent approaches such as 16S rRNA clone banks that showed the Betaproteobacteria dominated lab-scale EBPR reactors. Further work using clone banks and fluorescence in situ hybridization identified a group of bacteria, closely related to Rhodocyclus as the dominant member of lab-scale communities.

Rhamnolipids are a class of glycolipid produced by Pseudomonas aeruginosa, amongst other organisms, frequently cited as bacterial surfactants. They have a glycosyl head group, in this case a rhamnose moiety, and a 3-(hydroxyalkanoyloxy)alkanoic acid (HAA) fatty acid tail, such as 3-hydroxydecanoic acid.

In molecular biology, the OmpA domain is a conserved protein domain with a beta/alpha/beta/alpha-beta(2) structure found in the C-terminal region of many Gram-negative bacterial outer membrane proteins, such as porin-like integral membrane proteins, small lipid-anchored proteins, and MotB proton channels. The N-terminal half of these proteins is variable although some of the proteins in this group have the OmpA-like transmembrane domain at the N terminus. OmpA from Escherichia coli is required for pathogenesis, and can interact with host receptor molecules. MotB serve two functions in E. coli, the MotA(4)-MotB(2) complex attaches to the cell wall via MotB to form the stator of the flagellar motor, and the MotA-MotB complex couples the flow of ions across the cell membrane to movement of the rotor.
Alcohol dehydrogenase (cytochrome c) (EC 1.1.2.8, type I quinoprotein alcohol dehydrogenase, quinoprotein ethanol dehydrogenase) is an enzyme with systematic name alcohol:cytochrome c oxidoreductase. This enzyme catalyses the following chemical reaction

Robert Ernest William Hancock is a Canadian microbiologist and University of British Columbia Killam Professor of Microbiology and Immunology, an Associate Faculty Member of the Wellcome Trust Sanger Institute, and a Canada Research Chair in Health and Genomics.
References
- ↑ Hancock RE, Egli C, Benz R, Siehnel RJ (January 1992). "Overexpression in Escherichia coli and functional analysis of a novel PPi-selective porin, oprO, from Pseudomonas aeruginosa". J. Bacteriol. 174 (2): 471–6. doi:10.1128/jb.174.2.471-476.1992. PMC 205739 . PMID 1370289.
- ↑ Siehnel RJ, Egli C, Hancock RE (August 1992). "Polyphosphate-selective porin OprO of Pseudomonas aeruginosa: expression, purification and sequence". Mol. Microbiol. 6 (16): 2319–26. doi:10.1111/j.1365-2958.1992.tb01407.x. PMID 1406271. S2CID 23190468.