Related Research Articles

The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. It consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive element polonium (Po). The chemically uncharacterized synthetic element livermorium (Lv) is predicted to be a chalcogen as well. Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.

Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature.

Hydrogen sulfide is a chemical compound with the formula H
2S. It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The underground mine gas term for foul-smelling hydrogen sulfide-rich gas mixtures is stinkdamp. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. The British English spelling of this compound is hydrogen sulphide, a spelling no longer recommended by the Royal Society of Chemistry or the International Union of Pure and Applied Chemistry.

An organic sulfide or thioether is a functional group in organosulfur chemistry with the connectivity C–S–C as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application.

Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.
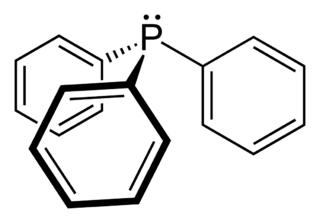
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

The Claus process is the most significant gas desulfurizing process, recovering elemental sulfur from gaseous hydrogen sulfide. First patented in 1883 by the chemist Carl Friedrich Claus, the Claus process has become the industry standard.
The oil and gas industry is usually divided into three major sectors: upstream, midstream, and downstream. The downstream sector is the refining of petroleum crude oil and the processing and purifying of raw natural gas, as well as the marketing and distribution of products derived from crude oil and natural gas. The downstream sector reaches consumers through products such as gasoline or petrol, kerosene, jet fuel, diesel oil, heating oil, fuel oils, lubricants, waxes, asphalt, natural gas, and liquefied petroleum gas (LPG) as well as naphtha and hundreds of petrochemicals.
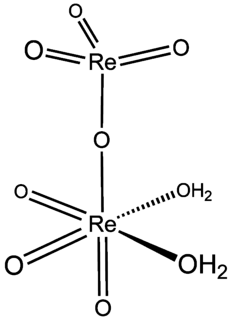
Perrhenic acid is the chemical compound with the formula Re
2O
7(OH
2)
2. It is obtained by evaporating aqueous solutions of Re
2O
7. Conventionally, perrhenic acid is considered to have the formula HReO
4, and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam. When a solution of Re
2O
7 is kept for a period of months, it breaks down and crystals of HReO
4·H
2O are formed, which contain tetrahedral ReO−
4 For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid.

The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a constituent of many proteins and cofactors, and sulfur compounds can be used as oxidants or reductants in microbial respiration. The global sulfur cycle involves the transformations of sulfur species through different oxidation states, which play an important role in both geological and biological processes. Steps of the sulfur cycle are:
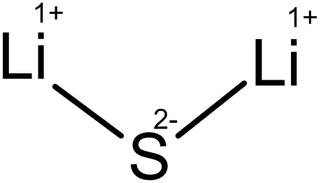
Lithium sulfide is the inorganic compound with the formula Li2S. It crystallizes in the antifluorite motif, described as the salt (Li+)2S2−. It forms a solid yellow-white deliquescent powder. In air, it easily hydrolyses to release hydrogen sulfide (rotten egg odor).
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to organic compounds, deoxygenation is a component of fuels production as well a type of reaction employed in organic synthesis, e.g. of pharmaceuticals.

Bismuth(III) sulfide is a chemical compound of bismuth and sulfur. It occurs in nature as the mineral bismuthinite.
Sulfur is metabolized by all organisms, from bacteria and archaea to plants and animals. Sulfur is reduced or oxidized by organisms in a variety of forms. The element is present in proteins, sulfate esters of polysaccharides, steroids, phenols, and sulfur-containing coenzymes.
CrystaSulf is the trade name for a chemical process used for removing hydrogen sulfide (H2S) from natural gas, synthesis gas and other gas streams in refineries and chemical plants. CrystaSulf uses a modified liquid-phase Claus reaction to convert the hydrogen sulfide (H2S) into elemental sulfur which is then removed from the process by filtration. CrystaSulf is used in the energy industry as a mid-range process to handle sulfur amounts between 0.1 and 20 tons per day. Below 0.1 tons of sulfur per day is typically managed by H2S Scavengers and applications above 20 tons per day are typically treated with the Amine – Claus process.

Chloro(dimethyl sulfide)gold(I) is a coordination complex of gold. It is a white solid. This compound is a common entry point into gold chemistry.

Trisulfane is the inorganic compound with the formula H2S3. It is a pale yellow volatile liquid with a camphor-like odor. It decomposes readily to hydrogen sulfide (H2S) and elemental sulfur. It is produced by distillation of the polysulfane oil obtained by acidification of polysulfide salts.

Sodium polysulfide is a general term for salts with the formula Na2Sx, where x = 2 to 5. The species Sx2−, called polysulfide anions, include disulfide (S22−), trisulfide (S32−), tetrasulfide (S42−), and pentasulfide (S52−). In principle, but not in practice, the chain lengths could be longer. The salts are dark red solids that dissolve in water to give highly alkaline and corrosive solutions. In air, these salts oxidize, and they evolve hydrogen sulfide by hydrolysis.

Tris(dimethylamino)phosphine is an organophosphorus compound with the formula P(NMe2)3 (Me = methyl). It is a colorless oil at room temperature, and is one of the most common aminophosphines. Its structure has been determined by X-ray crystallography.

Transition metal thioether complexes comprise coordination complexes of thioether (R2S) ligands. The inventory is extensive.
References
- ↑ Capps, Kenneth B.; Wixmerten, Bodo; Bauer, Andreas; Hoff, Carl D. (1998). "Thermochemistry of Sulfur Atom Transfer. Enthalpies of Reaction of Phosphines with Sulfur, Selenium, and Tellurium, and of Desulfurization of Triphenylarsenic Sulfide, Triphenylantimony Sulfide, and Benzyl Trisulfide". Inorganic Chemistry. 37 (12): 2861–2864. doi:10.1021/ic9715862.