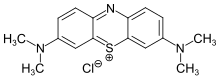
Redox is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction.

Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible (400–750 nm), or infrared radiation (750–2500 nm).

In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a photocatalyst, the excited state of which "repeatedly interacts with the reaction partners forming reaction intermediates and regenerates itself after each cycle of such interactions." In many cases, the catalyst is a solid that upon irradiation with UV- or visible light generates electron–hole pairs that generate free radicals. Photocatalysts belong to three main groups; heterogeneous, homogeneous, and plasmonic antenna-reactor catalysts. The use of each catalysts depends on the preferred application and required catalysis reaction.

In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks. Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.

Photosensitizers are light absorbers that alter the course of a photochemical reaction. They usually are catalysts. They can function by many mechanisms, sometimes they donate an electron to the substrate, sometimes they abstract a hydrogen atom from the substrate. At the end of this process, the photosensitizer returns to its ground state, where it remains chemically intact, poised to absorb more light. One branch of chemistry which frequently utilizes photosensitizers is polymer chemistry, using photosensitizers in reactions such as photopolymerization, photocrosslinking, and photodegradation. Photosensitizers are also used to generate prolonged excited electronic states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy. Generally, photosensitizers absorb electromagnetic radiation consisting of infrared radiation, visible light radiation, and ultraviolet radiation and transfer absorbed energy into neighboring molecules. This absorption of light is made possible by photosensitizers' large de-localized π-systems, which lowers the energy of HOMO and LUMO orbitals to promote photoexcitation. While many photosensitizers are organic or organometallic compounds, there are also examples of using semiconductor quantum dots as photosensitizers.

Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal catalyst. Polymerization from this method is called atom transfer radical addition polymerization (ATRAP). As the name implies, the atom transfer step is crucial in the reaction responsible for uniform polymer chain growth. ATRP was independently discovered by Mitsuo Sawamoto and by Krzysztof Matyjaszewski and Jin-Shan Wang in 1995.

In industrial chemistry, a stabilizer or stabiliser is a chemical that is used to prevent degradation.
Polymer stabilizers are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.

In polymer chemistry photo-oxidation is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break, resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering.
Photocatalytic water splitting is a process that uses photocatalysis for the dissociation of water (H2O) into hydrogen (H
2) and oxygen (O
2). The inputs are light energy (photons), water, and a catalyst(s). The process is inspired by Photosynthesis, which converts water and carbon dioxide into oxygen and carbohydrates. Water splitting using solar radiation has not been commercialized. Photocatalytic water splitting is done by dispersing photocatalyst particles in water or depositing them on a substrate, unlike Photoelectrochemical cell, which are assembled into a cell with a photoelectrode. Hydrogen fuel production using water and light (photocatalytic water splitting), instead of petroleum, is an important renewable energy strategy.
Jin-Shan Wang, is a Chinese-American organic chemist and entrepreneur.

Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "living free radical polymerization", though the two terms are not synonymous.
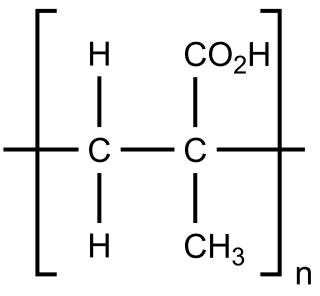
Poly(methacrylic acid) (PMAA) is a polymer made from methacrylic acid (preferred IUPAC name, 2-methylprop-2-enoic acid), which is a carboxylic acid. It is often available as its sodium salt, poly(methacrylic acid) sodium salt. The monomer is a viscous liquid with a pungent odour. The first polymeric form of methacrylic acid was described in 1880 by Engelhorn and Fittig. The use of high purity monomers is required for proper polymerization conditions and therefore it is necessary to remove any inhibitors by extraction (phenolic inhibitors) or via distillation. To prevent inhibition by dissolved oxygen, monomers should be carefully degassed prior to the start of the polymerization.

In polymer chemistry, reversible-deactivation radical polymerizations (RDRPs) are members of the class of reversible-deactivation polymerizations which exhibit much of the character of living polymerizations, but cannot be categorized as such as they are not without chain transfer or chain termination reactions. Several different names have been used in literature, which are:
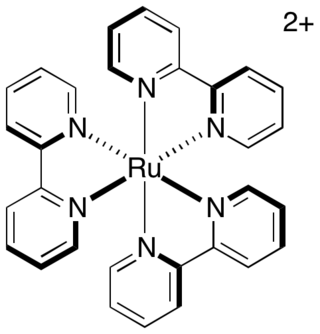
Photoredox catalysis is a branch of photochemistry that uses single-electron transfer. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes, and semiconductors. While organic photoredox catalysts were dominant throughout the 1990s and early 2000s, soluble transition-metal complexes are more commonly used today.

Automated synthesis or automatic synthesis is a set of techniques that use robotic equipment to perform chemical synthesis in an automated way. Automating processes allows for higher efficiency and product quality although automation technology can be cost-prohibitive and there are concerns regarding overdependence and job displacement. Chemical processes were automated throughout the 19th and 20th centuries, with major developments happening in the previous thirty years, as technology advanced. Tasks that are performed may include: synthesis in variety of different conditions, sample preparation, purification, and extractions. Applications of automated synthesis are found on research and industrial scales in a wide variety of fields including polymers, personal care, and radiosynthesis.

Single Chain Cyclized/Knotted Polymers are a new class of polymer architecture with a general structure consisting of multiple intramolecular cyclization units within a single polymer chain. Such a structure was synthesized via the controlled polymerization of multivinyl monomers, which was first reported in Dr. Wenxin Wang's research lab. These multiple intramolecular cyclized/knotted units mimic the characteristics of complex knots found in proteins and DNA which provide some elasticity to these structures. Of note, 85% of elasticity in natural rubber is due to knot-like structures within its molecular chain.
An intramolecular cyclization reaction is where the growing polymer chain reacts with a vinyl functional group on its own chain, rather than with another growing chain in the reaction system. In this way the growing polymer chain covalently links to itself in a fashion similar to that of a knot in a piece of string. As such, single chain cyclized/knotted polymers consist of many of these links, as opposed to other polymer architectures including branched and crosslinked polymers that are formed by two or more polymer chains in combination.
Copper-based reversible-deactivation radical polymerization(Cu-based RDRP) is a member of the class of reversible-deactivation radical polymerization. In this system, various copper species are employed as the transition-metal catalyst for reversible activation/deactivation of the propagating chains responsible for uniform polymer chain growth.
Photopolymerization-based signal amplification (PBA) is a method of amplifying detection signals from molecular recognition events in an immunoassay by utilizing a radical polymerization initiated through illumination by light. To contrast between a negative and a positive result, PBA is linked to a colorimetric method, thereby resulting in a change in color when a targeted analyte is detected, i.e., a positive signal. PBA is also used to quantify the concentration of the analyte by measuring intensity of the color.
Mitsuo Sawamoto is a Japanese chemist specializing in the field of polymer chemistry, Emeritus Professor at Kyoto University, professor at Chubu University.