Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.
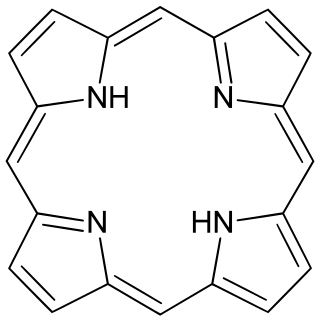
Porphyrins are a group of heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges. In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis.
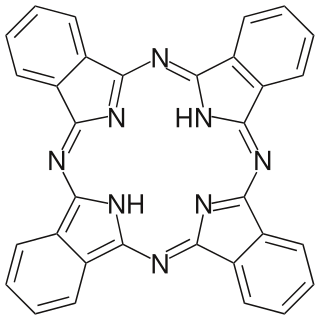
Phthalocyanine is a large, aromatic, macrocyclic, organic compound with the formula (C8H4N2)4H2 and is of theoretical or specialized interest in chemical dyes and photoelectricity.
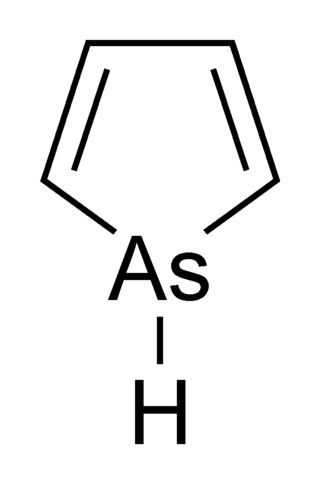
Arsole, also called arsenole or arsacyclopentadiene, is an organoarsenic compound with the formula C4H4AsH. It is classified as a metallole and is isoelectronic to and related to pyrrole except that an arsenic atom is substituted for the nitrogen atom. Whereas the pyrrole molecule is planar, the arsole molecule is not, and the hydrogen atom bonded to arsenic extends out of the molecular plane. Arsole is only moderately aromatic, with about 40% the aromaticity of pyrrole. Arsole itself has not been reported in pure form, but several substituted analogs called arsoles exist. Arsoles and more complex arsole derivatives have similar structure and chemical properties to those of phosphole derivatives. When arsole is fused to a benzene ring, this molecule is called arsindole, or benzarsole.

A corrole is an aromatic tetrapyrrole. The corrin ring is also present in cobalamin (vitamin B12). The ring consists of nineteen carbon atoms, with four nitrogen atoms in the core of the molecule. In this sense, corrole is very similar to porphyrin.

(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.

Porphine or porphin is an organic compound of empirical formula C20H14N4. It is heterocyclic and aromatic. The molecule is a flat macrocycle, consisting of four pyrrole-like rings joined by four methine bridges, which makes it the simplest of the tetrapyrroles.

Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not soluble in water. The name is often abbreviated as PPIX.
In organic chemistry, bilane is a compound with the formula C19H20N4 or [(C4H4N)−CH2−(C4H3N)−]2CH2. It is a tetrapyrrole, a class of compounds with four independent pyrrole rings. Specifically, the molecule can be described as four pyrrole molecules C4H5N connected in an open chain by three methylene bridges −CH2− at carbons adjacent to the nitrogens, replacing the respective hydrogens.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

The Rothemund reaction is a condensation/oxidation process that converts four pyrroles and four aldehydes into a porphyrin. It is based on work by Paul Rothemund, who first reported it in 1936. The method underpins more modern synthesis such as those described by Adler and Longo and by Lindsey. The Rothemund reactions is common in university teaching labs.
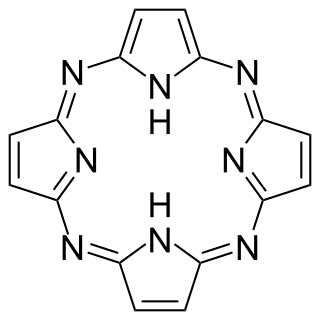
Porphyrazines, or tetraazaporphyrins, are tetrapyrrole macrocycles similar to porphyrins and phthalocyanines. Pioneered by Sir R. Patrick Linstead as an extension of his work on phthalocyanines, porphyrazines differ from porphyrins in that they contain -meso nitrogen atoms, rather than carbon atoms, and differ from phthalocyanines in that their β-pyrrole positions are open for substitution. These differences confer physical properties that are distinct from both porphyrins and phthalocyanines.
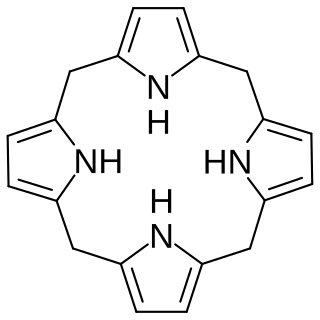
In biochemistry, a porphyrinogen is a member of a class of naturally occurring compounds with a tetrapyrrole core, a macrocycle of four pyrrole rings connected by four methylene bridges. They can be viewed as derived from the parent compound hexahydroporphine by the substitution of various functional groups for hydrogen atoms in the outermost (20-carbon) ring.
Boron porphyrins are a variety of porphyrin, a common macrocycle used for photosensitization and metal trapping applications, that incorporate boron. The central four nitrogen atoms in a porphyrin macrocycle form a unique molecular pocket which is known to accommodate transition metals of various sizes and oxidation states. Due to the diversity of binding modes available to porphyrin, there is a growing interest in introducing other elements into this pocket.
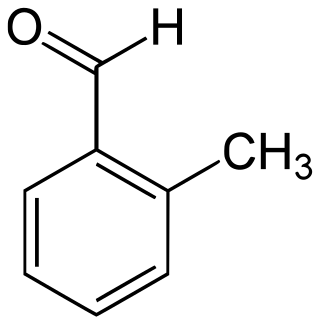
2-Methylbenzaldehyde is an organic compound with the formula CH3C6H4CHO. It is a colorless liquid.
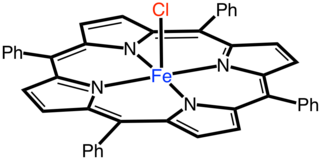
Iron(tetraporphyrinato) chloride is the coordination complex with the formula Fe(TPP)Cl where TPP is the dianion [C44H28N4]2-. The compound forms blue microcrystals that dissolve in chlorinated solvent to give brown solutions. In terms of structure, the complex is five-coordinate with idealized C4v point group symmetry. It is one of more common transition metal porphyrin complexes.

meso-Octamethylporphyrinogen, usually referred to simply as octamethylporphyrinogen, is an organic compound with the formula (Me2C-C4H2NH)4 (Me = CH3. It is one of the simplest porphyrinogens, a family of compounds that occur as intermediates in the biosynthesis of hemes and chlorophylls. In contrast to those rings, porphyrinogens are colorless since they lack extended conjugation. The prefix meso-octamethyl indicates that the eight methyl groups are located on the carbon centers that interconnect the four pyrrole rings. Also unlike porphyrins, the porphyrinogens are highly ruffled.

Transition metal porphyrin complexes are a family of coordination complexes of the conjugate base of porphyrins. Iron porphyrin complexes occur widely in Nature, which has stimulated extensive studies on related synthetic complexes. The metal-porphyrin interaction is a strong one such that metalloporphyrins are thermally robust. They are catalysts and exhibit rich optical properties, although these complexes remain mainly of academic interest.

Phosphorus-centered porphyrins are conjugated polycyclic ring systems consisting of either four pyrroles with inward-facing nitrogens and a phosphorus atom at their core or porphyrins with one of the four pyrroles substituted for a phosphole. Unmodified porphyrins are composed of pyrroles and linked by unsaturated hydrocarbon bridges often acting as multidentate ligands centered around a transition metal like Cu II, Zn II, Co II, Fe III. Being highly conjugated molecules with many accessible energy levels, porphyrins are used in biological systems to perform light-energy conversion and modified synthetically to perform similar functions as a photoswitch or catalytic electron carriers. Phosphorus III and V ions are much smaller than the typical metal centers and bestow distinct photochemical properties unto the porphyrin. Similar compounds with other pnictogen cores or different polycyclic rings coordinated to phosphorus result in other changes to the porphyrin’s chemistry.




















