Related Research Articles
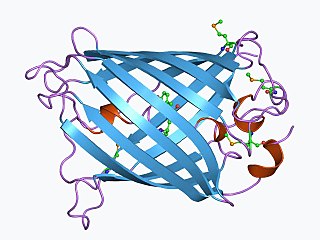
The green fluorescent protein (GFP) is a protein that exhibits bright green fluorescence when exposed to light in the blue to ultraviolet range. The label GFP traditionally refers to the protein first isolated from the jellyfish Aequorea victoria and is sometimes called avGFP. However, GFPs have been found in other organisms including corals, sea anemones, zoanithids, copepods and lancelets.
Cameleon is an engineered protein based on variant of green fluorescent protein used to visualize calcium levels in living cells. It is a genetically encoded calcium sensor created by Roger Y. Tsien and coworkers. The name is a conflation of CaM and chameleon to indicate the fact that the sensor protein undergoes a conformation change and radiates at an altered wavelength upon calcium binding to the calmodulin element of the Cameleon. Cameleon was the first genetically encoded calcium sensor that could be used for ratiometric measurements and the first to be used in a transgenic animal to record activity in neurons and muscle cells. Cameleon and other genetically-encoded calcium indicators (GECIs) have found many applications in neuroscience and other fields of biology. It was created by fusing BFP, calmodulin, calmodulin-binding peptide M13 and EGFP.

A degron is a portion of a protein that is important in regulation of protein degradation rates. Known degrons include short amino acid sequences, structural motifs and exposed amino acids located anywhere in the protein. In fact, some proteins can even contain multiple degrons. Degrons are present in a variety of organisms, from the N-degrons first characterized in yeast to the PEST sequence of mouse ornithine decarboxylase. Degrons have been identified in prokaryotes as well as eukaryotes. While there are many types of different degrons, and a high degree of variability even within these groups, degrons are all similar for their involvement in regulating the rate of a protein's degradation. Much like protein degradation mechanisms are categorized by their dependence or lack thereof on Ubiquitin, a small protein involved in proteasomal protein degradation, Degrons may also be referred to as “Ubiquitin-dependent" or “Ubiquitin-independent".
Kaede is a photoactivatable fluorescent protein naturally originated from a stony coral, Trachyphyllia geoffroyi. Its name means "maple" in Japanese. With the irradiation of ultraviolet light (350–400 nm), Kaede undergoes irreversible photoconversion from green fluorescence to red fluorescence.
EosFP is a photoactivatable green to red fluorescent protein. Its green fluorescence (516 nm) switches to red (581 nm) upon UV irradiation of ~390 nm due to a photo-induced modification resulting from a break in the peptide backbone near the chromophore. Eos was first discovered as a tetrameric protein in the stony coral Lobophyllia hemprichii. Like other fluorescent proteins, Eos allows for applications such as the tracking of fusion proteins, multicolour labelling and tracking of cell movement. Several variants of Eos have been engineered for use in specific study systems including mEos2, mEos4 and CaMPARI.

Jennifer Lippincott-Schwartz is a Senior Group Leader at Howard Hughes Medical Institute's Janelia Research Campus and a founding member of the Neuronal Cell Biology Program at Janelia. Previously, she was the Chief of the Section on Organelle Biology in the Cell Biology and Metabolism Program, in the Division of Intramural Research in the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health from 1993 to 2016. Lippincott-Schwartz received her Ph.D. from Johns Hopkins University, and performed post-doctoral training with Dr. Richard Klausner at the NICHD, NIH in Bethesda, Maryland.

8-Bromoguanosine 3′,5′-cyclic monophosphate is a brominated derivative of cyclic guanosine monophosphate (cGMP). It acts as an activator of cGMP-dependent protein kinases.
A chromoprotein is a conjugated protein that contains a pigmented prosthetic group. A common example is haemoglobin, which contains a heme cofactor, which is the iron-containing molecule that makes oxygenated blood appear red. Other examples of chromoproteins include other hemochromes, cytochromes, phytochromes and flavoproteins.
mCherry is a member of the mFruits family of monomeric red fluorescent proteins (mRFPs). As a RFP, mCherry was derived from DsRed of Discosoma sea anemones unlike green fluorescent proteins (GFPs) which are often derived from Aequoera victoria jellyfish. Fluorescent proteins are used to tag components in the cell, so they can be studied using fluorescence spectroscopy and fluorescence microscopy. mCherry absorbs light between 540-590 nm and emits light in the range of 550-650 nm. mCherry belongs to the group of fluorescent protein chromophores used as instruments to visualize genes and analyze their functions in experiments. Genome editing has been improved greatly through the precise insertion of these fluorescent protein tags into the genetic material of many diverse organisms. Most comparisons between the brightness and photostability of different fluorescent proteins have been made in vitro, removed from biological variables that affect protein performance in cells or organisms. It is hard to perfectly simulate cellular environments in vitro, and the difference in environment could have an effect on the brightness and photostability.
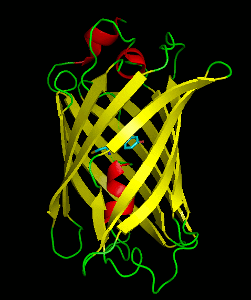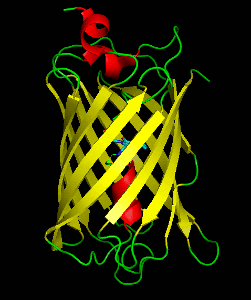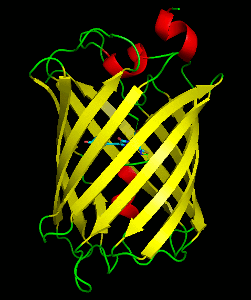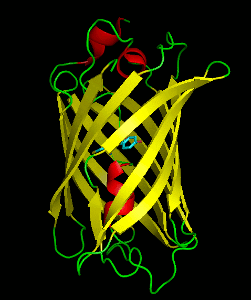
Dronpa is a reversibly switchable photoactivatable fluorescent protein that is 2.5 times as bright as EGFP. Dronpa gets switched off by strong illumination with 488 nm (blue) light and this can be reversed by weak 405 nm UV light. A single dronpa molecule can be switched on and off over 100 times. It has an excitation peak at 503 nm and an emission peak at 518 nm.

Photoactivatable probes, or caged probes, are cellular players that can be triggered by a flash of light. They are used in biological research to study processes in cells. The basic principle is to bring a photoactivatable agent to cells, tissues or even living animals and specifically control its activity by illumination.
Calcium imaging is a microscopy technique to optically measure the calcium (Ca2+) status of an isolated cell, tissue or medium. Calcium imaging takes advantage of calcium indicators, fluorescent molecules that respond to the binding of Ca2+ ions by fluorescence properties. Two main classes of calcium indicators exist: chemical indicators and genetically encoded calcium indicators (GECI). This technique has allowed studies of calcium signalling in a wide variety of cell types. In neurons, electrical activity is always accompanied by an influx of Ca2+ ions. Thus, calcium imaging can be used to monitor the electrical activity in hundreds of neurons in cell culture or in living animals, which has made it possible to dissect the function of neuronal circuits.
David M. Knipe is the Higgins Professor of Microbiology and Molecular Genetics and interim Co-Chair in the Department of Microbiology and Immunobiology at the Harvard Medical School in Boston, Massachusetts and co-chief editor of the reference book Fields Virology. He had previously served as Chair of the Program in Virology at Harvard Medical School from 2004 through 2016.
BEAMing, which stands for beads, emulsion, amplification, magnetics, is a highly sensitive digital PCR method that combines emulsion PCR and flow cytometry to identify and quantify specific somatic mutations present in DNA.
A genetically engineered fluorescent protein that changes its fluorescence when bound to the neurotransmitter glutamate. Glutamate-sensitive fluorescent reporters are used to monitor the activity of presynaptic terminals by fluorescence microscopy. GluSnFRs are a class of optogenetic sensors used in neuroscience research. In brain tissue, two-photon microscopy is typically used to monitor GluSnFR fluorescence.

Red fluorescent protein (RFP) is a fluorophore that fluoresces red-orange when excited. Several variants have been developed using directed mutagenesis. The original was isolated from Discosoma, and named DsRed. Others are now available that fluoresce orange, red, and far-red.

Marcos Boris Rotman was a Chilean American immunologist–molecular biologist and professor emeritus of Medical Science at Alpert Medical School of Brown University. He is widely recognized for performing the first single molecule experiments in biology. He died in July 2021 at the age of 96.
FAST is a small, genetically-encoded, protein tag which allows for fluorescence reporting of proteins of interest. Unlike natural fluorescent proteins and derivates such as GFP or mCherry, FAST is not fluorescent by itself. It can bind selectively a fluorogenic chromophore derived from 4-hydroxybenzylidene rhodanine (HBR), which is itself non fluorescent unless bound. Once bound, the pair of molecules goes through a unique fluorogen activation mechanism based on two spectroscopic changes, increase of fluorescence quantum yield and absorption red shift, hence providing high labeling selectivity. The FAST-fluorogen reporting system can be used in fluorescence microscopy, flow cytometry and any other fluorometric method to explore the living world: biosensors, protein trafficking.
Charles Clifton Richardson is an American biochemist and professor at Harvard University. Richardson received his undergraduate education at Duke University, where he majored in medicine. He received his M.D. at Duke Medical School in 1960. Richardson works as a professor at Harvard Medical School, and he served as editor/associate editor of the Annual Review of Biochemistry from 1972 to 2003. Richardson received the American Chemical Society Award in Biological Chemistry in 1968, as well as numerous other accolades.

Suliana Manley is an American biophysicist. Her research focuses on the development of high-resolution optical instruments, and their application in studying the organization and dynamics of proteins. She is an associate professor at École Polytechnique Fédérale de Lausanne and heads the Laboratory of Experimental Biophysics.
References
- 1 2 3 Ando, Ryoko; Hama, Hiroshi; Yamamoto-Hino, Miki; Mizuno, Hideaki; Miyawaki, Atsushi (2002-10-01). "An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein". Proceedings of the National Academy of Sciences. 99 (20): 12651–12656. Bibcode:2002PNAS...9912651A. doi: 10.1073/pnas.202320599 . ISSN 0027-8424. PMC 130515 . PMID 12271129.
- ↑ Lukyanov, Konstantin A.; Chudakov, Dmitry M.; Lukyanov, Sergey; Verkhusha, Vladislav V. (November 2005). "Photoactivatable fluorescent proteins". Nature Reviews Molecular Cell Biology. 6 (11): 885–890. doi:10.1038/nrm1741. ISSN 1471-0072. PMID 16167053. S2CID 23734846.
- ↑ Wiedenmann, J.; Ivanchenko, S.; Oswald, F.; Schmitt, F.; Rocker, C.; Salih, A.; Spindler, K.-D.; Nienhaus, G. U. (2004-11-09). "EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion". Proceedings of the National Academy of Sciences. 101 (45): 15905–15910. Bibcode:2004PNAS..10115905W. doi: 10.1073/pnas.0403668101 . ISSN 0027-8424. PMC 528746 . PMID 15505211.
- ↑ Adam, Virgile; Lelimousin, Mickaël; Boehme, Susan; Desfonds, Guillaume; Nienhaus, Karin; Field, Martin J.; Wiedenmann, Joerg; McSweeney, Sean; Nienhaus, G. Ulrich; Bourgeois, Dominique (2008-11-25). "Structural characterization of IrisFP, an optical highlighter undergoing multiple photo-induced transformations". Proceedings of the National Academy of Sciences. 105 (47): 18343–18348. Bibcode:2008PNAS..10518343A. doi: 10.1073/pnas.0805949105 . ISSN 0027-8424. PMC 2587625 . PMID 19017808.
- ↑ Tsutsui, Hidekazu; Karasawa, Satoshi; Shimizu, Hideaki; Nukina, Nobuyuki; Miyawaki, Atsushi (March 2005). "Semi‐rational engineering of a coral fluorescent protein into an efficient highlighter". EMBO Reports. 6 (3): 233–238. doi:10.1038/sj.embor.7400361. ISSN 1469-221X. PMC 1299271 . PMID 15731765.
- ↑ Habuchi, Satoshi; Ando, Ryoko; Dedecker, Peter; Verheijen, Wendy; Mizuno, Hideaki; Miyawaki, Atsushi; Hofkens, Johan (2005-07-05). "Reversible single-molecule photoswitching in the GFP-like fluorescent protein Dronpa". Proceedings of the National Academy of Sciences. 102 (27): 9511–9516. Bibcode:2005PNAS..102.9511H. doi: 10.1073/pnas.0500489102 . ISSN 0027-8424. PMC 1157093 . PMID 15972810.
- ↑ Patterson, George H; Lippincott-Schwartz, Jennifer (April 2004). "Selective photolabeling of proteins using photoactivatable GFP". Methods. 32 (4): 445–450. doi:10.1016/j.ymeth.2003.10.006. PMID 15003607.
- ↑ Chudakov, Dmitriy M; Verkhusha, Vladislav V; Staroverov, Dmitry B; Souslova, Ekaterina A; Lukyanov, Sergey; Lukyanov, Konstantin A (2004-11-01). "Photoswitchable cyan fluorescent protein for protein tracking". Nature Biotechnology. 22 (11): 1435–1439. doi:10.1038/nbt1025. ISSN 1087-0156. PMID 15502815. S2CID 9597405.