
Staphylococcus aureus is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe that can grow without the need for oxygen. Although S. aureus usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. S. aureus is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA), is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved.

Methicillin-resistant Staphylococcus aureus (MRSA) is a group of gram-positive bacteria that are genetically distinct from other strains of Staphylococcus aureus. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths attributable to antimicrobial resistance in 2019.

Staphylococcus xylosus is a species of bacteria belonging to the genus Staphylococcus. It is a Gram-positive bacterium that forms clusters of cells. Like most staphylococcal species, it is coagulase-negative and exists as a commensal on the skin of humans and animals and in the environment.

Dicloxacillin is a narrow-spectrum β-lactam antibiotic of the penicillin class. It is used to treat infections caused by susceptible (non-resistant) Gram-positive bacteria. It is active against beta-lactamase-producing organisms such as Staphylococcus aureus, which would otherwise be resistant to most penicillins. Dicloxacillin is available under a variety of trade names including Diclocil (BMS).

Panton–Valentine leukocidin (PVL) is a cytotoxin—one of the β-pore-forming toxins. The presence of PVL is associated with increased virulence of certain strains (isolates) of Staphylococcus aureus. It is present in the majority of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) isolates studied and is the cause of necrotic lesions involving the skin or mucosa, including necrotic hemorrhagic pneumonia. PVL creates pores in the membranes of infected cells. PVL is produced from the genetic material of a bacteriophage that infects Staphylococcus aureus, making it more virulent.
Lysostaphin is a Staphylococcus simulans metalloendopeptidase. It can function as a bacteriocin (antimicrobial) against Staphylococcus aureus.

Ceftobiprole (Zevtera/Mabelio) is a fifth-generation cephalosporin for the treatment of hospital-acquired pneumonia and community-acquired pneumonia. It is marketed by Basilea Pharmaceutica in the United Kingdom, Germany, Switzerland and Austria under the trade name Zevtera, in France and Italy under the trade name Mabelio. Like other cephalosporins, ceftobiprole exerts its antibacterial activity by binding to important penicillin-binding proteins and inhibiting their transpeptidase activity which is essential for the synthesis of bacterial cell walls. Ceftobiprole has high affinity for penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus strains and retains its activity against strains that express divergent mecA gene homologues. Ceftobiprole also binds to penicillin-binding protein 2b in Streptococcus pneumoniae (penicillin-intermediate), to penicillin-binding protein 2x in Streptococcus pneumoniae (penicillin-resistant), and to penicillin-binding protein 5 in Enterococcus faecalis.
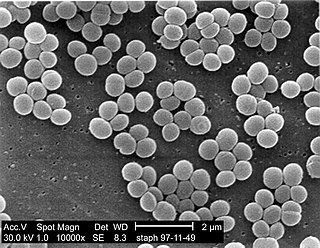
A staphylococcal infection or staph infection is an infection caused by members of the Staphylococcus genus of bacteria.
mecA is a gene found in bacterial cells which allows them to be resistant to antibiotics such as methicillin, penicillin and other penicillin-like antibiotics.

Staphylococcus capitis is a coagulase-negative species (CoNS) of Staphylococcus. It is part of the normal flora of the skin of the human scalp, face, neck, scrotum, and ears and has been associated with prosthetic valve endocarditis, but is rarely associated with native valve infection.

Staphylococcus is a genus of Gram-positive bacteria in the family Staphylococcaceae from the order Bacillales. Under the microscope, they appear spherical (cocci), and form in grape-like clusters. Staphylococcus species are facultative anaerobic organisms.
SaPIs are a family of ~15 kb mobile genetic elements resident in the genomes of the vast majority of S. aureus strains. Much like bacteriophages, SaPIs can be transferred to uninfected cells and integrate into the host chromosome. Unlike the bacterial viruses, however, integrated SaPIs are mobilized by host infection with "helper" bacteriophages. SaPIs are used by the host bacteria to co-opt the phage reproduction cycle for their own genetic transduction and also inhibit phage reproduction in the process.

Staphylococcus hyicus is a Gram-positive, facultatively anaerobic bacterium in the genus Staphylococcus. It consists of clustered cocci and forms white circular colonies when grown on blood agar. S. hyicus is a known animal pathogen. It causes disease in poultry, cattle, horses, and pigs. Most notably, it is the agent that causes porcine exudative epidermitis, also known as greasy pig disease, in piglets. S. hyicus is generally considered to not be zoonotic, however it has been shown to be able to cause bacteremia and sepsis in humans.

Enzybiotics are an experimental antibacterial therapy first described by Nelson, Loomis, and Fischetti. The term is derived from a combination of the words “enzyme” and “antibiotics.” Enzymes have been extensively utilized for their antibacterial and antimicrobial properties. Proteolytic enzymes called endolysins have demonstrated particular effectiveness in combating a range of bacteria and are the basis for enzybiotic research. Endolysins are derived from bacteriophages and are highly efficient at lysing bacterial cells. Enzybiotics are being researched largely to address the issue of antibiotic resistance, which has allowed for the proliferation of drug-resistant pathogens posing great risk to animal and human health across the globe.
Mary Barber was a British pathologist and bacteriologist who studied antibiotic resistance in bacteria. She was one of the pioneers in this field, documenting the phenomenon of penicillin resistance early on.
Staphylococcus schleiferi is a Gram-positive, cocci-shaped bacterium of the family Staphylococcaceae. It is facultatively anaerobic, coagulase-variable, and can be readily cultured on blood agar where the bacterium tends to form opaque, non-pigmented colonies and beta (β) hemolysis. There exists two subspecies under the species S. schleiferi: Staphylococcus schleiferi subsp. schleiferi and Staphylococcus schleiferi subsp. coagulans.
Staphylococcus pseudintermedius is a gram positive coccus bacteria of the genus Staphylococcus found worldwide. It is primarily a pathogen for domestic animals, but has been known to affect humans as well. S. pseudintermedius is an opportunistic pathogen that secretes immune modulating virulence factors, has many adhesion factors, and the potential to create biofilms, all of which help to determine the pathogenicity of the bacterium. Diagnoses of Staphylococcus pseudintermedius have traditionally been made using cytology, plating, and biochemical tests. More recently, molecular technologies like MALDI-TOF, DNA hybridization and PCR have become preferred over biochemical tests for their more rapid and accurate identifications. This includes the identification and diagnosis of antibiotic resistant strains.

Karen M. Frank is an American clinical pathologist and microbiologist researching the pathogenesis of Staphylococcus aureus pneumonia and resistant gram-negative bacteria. She is a senior clinician, principal investigator, and chief of laboratory medicine at the National Institutes of Health Clinical Center.
Vincent A. Fischetti is a world renowned American microbiologist and immunologist. He is Professor of and Head of the Laboratory of Bacterial Pathogenesis and Immunology at Rockefeller University in New York City. His Laboratory is the oldest continuous laboratory at Rockefeller that started in 1926 and headed by 4 leading scientists over its near 100 year history: Homer Swift, Maclyn McCarty, Emil Gotschlich and now Vincent Fischetti. Keeping with the historical theme of infectious diseases, Fischetti's primary areas of research are bacterial pathogenesis, bacterial genomics, immunology, virology, microbiology, and therapeutics. He was the first scientist to clone and sequence a surface protein on gram-positive bacteria, the M protein from S. pyogenes, and determine its unique coiled-coil structure. He also was the first use phage lysins as a therapeutic and an effective alternative to conventional antibiotics.
John Edward Blair was an American bacteriologist and serologist. He was the president of the American Society for Microbiology in 1962.












