
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most common plastic. As of 2017, over 100 million tonnes of polyethylene resins are produced annually, accounting for 34% of the total plastics market. Its primary use is in packaging (plastic bags, plastic films, geomembranes, containers including bottles, etc.). Many kinds of polyethylene are known, with most having the chemical formula (C2H4)n. PE is usually a mixture of similar polymers of ethylene with various values of n. Polyethylene is a thermoplastic; however, it can become a thermoset plastic when modified (such as cross-linked polyethylene).

Succinic acid is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. The name derives from Latin succinum, meaning amber. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological roles as a metabolic intermediate being converted into fumarate by the enzyme succinate dehydrogenase in complex 2 of the electron transport chain which is involved in making ATP, and as a signaling molecule reflecting the cellular metabolic state. It is marketed as food additive E363. Succinate is generated in mitochondria via the tricarboxylic acid cycle (TCA), an energy-yielding process shared by all organisms. Succinate can exit the mitochondrial matrix and function in the cytoplasm as well as the extracellular space, changing gene expression patterns, modulating epigenetic landscape or demonstrating hormone-like signaling. As such, succinate links cellular metabolism, especially ATP formation, to the regulation of cellular function. Dysregulation of succinate synthesis, and therefore ATP synthesis, happens in some genetic mitochondrial diseases, such as Leigh syndrome, and Melas syndrome, and degradation can lead to pathological conditions, such as malignant transformation, inflammation and tissue injury.

Polyethylene glycol (PEG; ) is a polyether compound with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H−(O−CH2−CH2)n−OH.

A lithium polymer battery, or more correctly lithium-ion polymer battery, is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. High conductivity semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types and are used in applications where weight is a critical feature, like mobile devices and radio-controlled aircraft.
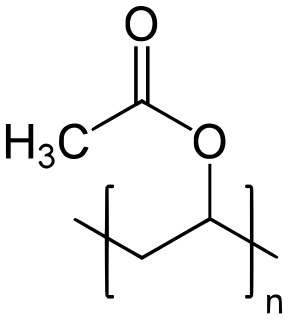
Poly(vinyl acetate) (PVA, PVAc, poly(ethenyl ethanoate): best known as wood glue, white glue, carpenter's glue, school glue, Elmer's glue in the US, or PVA glue) is an aliphatic rubbery synthetic polymer with the formula (C4H6O2)n. It belongs to the polyvinyl esters family, with the general formula -[RCOOCHCH2]-. It is a type of thermoplastic. There is considerable confusion between the glue as purchased, an aqueous emulsion of mostly vinyl acetate monomer, and the subsequent dried and polymerized PVAc that is the true thermoplastic polymer.
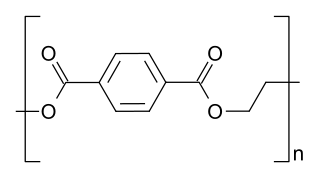
Polyethylene terephthalate, commonly abbreviated PET, PETE, or the obsolete PETP or PET-P, is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, thermoforming for manufacturing, and in combination with glass fibre for engineering resins.
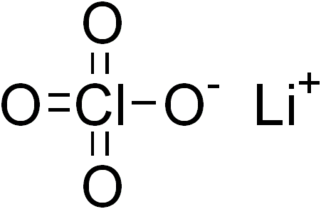
Lithium perchlorate is the inorganic compound with the formula LiClO4. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate.

Polyethylene naphthalate (poly is a polyester derived from naphthalene-2,6-dicarboxylic acid and ethylene glycol. As such it is related to poly, but with superior barrier properties.
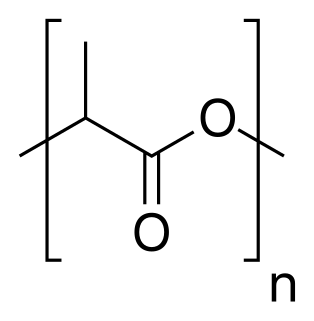
Poly(lactic acid) or polylactic acid or polylactide (PLA) is a biodegradable thermoplastic aliphatic polyester derived from renewable biomass, typically from fermented plant starch such as from corn, cassava, sugarcane or sugar beet pulp. In 2010, PLA had the second highest consumption volume of any bioplastic of the world.
Polysulfones are a family of thermoplastic polymers. These polymers are known for their toughness and stability at high temperatures. They contain the subunit aryl-SO2-aryl, the defining feature of which is the sulfone group. Polysulfones were introduced in 1965 by Union Carbide. Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.
Polydioxanone or poly-p-dioxanone is a colorless, crystalline, biodegradable synthetic polymer.
Poloxamers are nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene flanked by two hydrophilic chains of polyoxyethylene. The word poloxamer was coined by the inventor, Irving Schmolka, who received the patent for these materials in 1973. Poloxamers are also known by the trade names Synperonics, Pluronics, and Kolliphor.

Lithium succinate, the lithium salt of succinic acid, is a drug used in the treatment of seborrhoeic dermatitis and proposed for the treatment of anogenital warts.

In enzymology, a succinate-semialdehyde dehydrogenase (SSADH) (EC 1.2.1.24) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-(acetamidomethylene)succinate hydrolase (EC 3.5.1.29) is an enzyme that catalyzes the chemical reaction

Photo-oxidation is the degradation of a polymer surface in the presence of oxygen or ozone. The effect is facilitated by radiant energy such as UV or artificial light. This process is the most significant factor in weathering of polymers. Photo-oxidation is a chemical change that reduces the polymer's molecular weight. As a consequence of this change the material becomes more brittle, with a reduction in its tensile, impact and elongation strength. Discoloration and loss of surface smoothness accompany photo-oxidation. High temperature and localized stress concentrations are factors that significantly increase the effect of photo-oxidation.
Phosphinimide ligands, also known as phosphorane iminato ligands, are any of a class of organic compounds of the general formula NPR3−.

The gab operon is responsible for the conversion of γ-aminobutyrate (GABA) to succinate. The gab operon comprises three structural genes – gabD, gabT and gabP – that encode for a succinate semialdehyde dehydrogenase, GABA transaminase and a GABA permease respectively. There is a regulatory gene csiR, downstream of the operon, that codes for a putative transcriptional repressor and is activated when nitrogen is limiting.

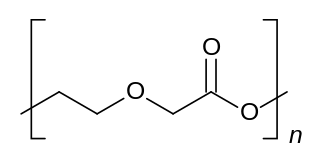
Polybutylene succinate (PBS) is a thermoplastic polymer resin of the polyester family. PBS is a biodegradable aliphatic polyester with properties that are comparable to polypropylene.
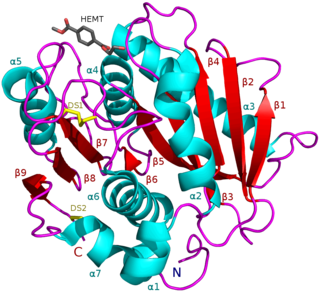
PETases are an esterase class of enzymes that catalyze the hydrolysis of poly (ethylene terephthalate) PET plastic to monomeric mono-2-hydroxyethyl terephthalate (MHET). The idealized chemical reaction is (where n is the number of monomers in the polymer chain):



















