
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradation occurs under a specific set of circumstances.

Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, and coatings, adhesives, electrical potting compounds, and fibers such as spandex and polyurethane laminate (PUL). Foams are the largest application accounting for 67% of all polyurethane produced in 2016.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Polybutylene terephthalate (PBT) is a thermoplastic engineering polymer that is used as an insulator in the electrical and electronics industries. It is a thermoplastic (semi-)crystalline polymer, and a type of polyester. PBT resists solvents, shrinks very little during forming, is mechanically strong, is heat-resistant up to 150 °C, and can be treated with flame retardants to make it noncombustible. It was developed by Britain's Imperial Chemical Industries (ICI).

Although PET is used in several applications, as of 2022 only bottles are collected at a substantial scale. The main motivations have been either cost reduction or recycle content of retail goods. An increasing amount is recycled back into bottles, the rest goes into fibres, film, thermoformed packaging and strapping. After sorting, cleaning and grinding, 'bottle flake' is obtained, which is then processed by either:

Polyhydroxyalkanoates or PHAs are polyesters produced in nature by numerous microorganisms, including through bacterial fermentation of sugars or lipids. When produced by bacteria they serve as both a source of energy and as a carbon store. More than 150 different monomers can be combined within this family to give materials with extremely different properties. These plastics are biodegradable and are used in the production of bioplastics.
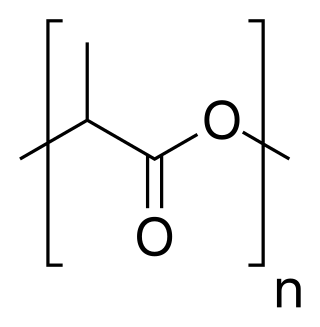
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.

Bioplastics are plastic materials produced from renewable biomass sources, such as vegetable fats and oils, corn starch, straw, woodchips, sawdust, recycled food waste, etc. Some bioplastics are obtained by processing directly from natural biopolymers including polysaccharides and proteins, while others are chemically synthesised from sugar derivatives and lipids from either plants or animals, or biologically generated by fermentation of sugars or lipids. In contrast, common plastics, such as fossil-fuel plastics are derived from petroleum or natural gas.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

Biodegradable plastics are plastics that can be decomposed by the action of living organisms, usually microbes, into water, carbon dioxide, and biomass. Biodegradable plastics are commonly produced with renewable raw materials, micro-organisms, petrochemicals, or combinations of all three.
Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases (CO2, N2), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether functional groups. Their properties and breakdown mechanism are determined by their exact structure. These polymers are often synthesized by condensation reactions, ring opening polymerization, and metal catalysts. There are vast examples and applications of biodegradable polymers.

Cyclohexanedimethanol (CHDM) is a mixture of isomeric organic compounds with formula C6H10(CH2OH)2. It is a colorless low-melting solid used in the production of polyester resins. Commercial samples consist of a mixture of cis and trans isomers. It is a di-substituted derivative of cyclohexane and is classified as a diol, meaning that it has two OH functional groups. Commercial CHDM typically has a cis/trans ratio of 30:70.

Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), commonly known as PHBV, is a polyhydroxyalkanoate-type polymer. It is biodegradable, nontoxic, biocompatible plastic produced naturally by bacteria and a good alternative for many non-biodegradable synthetic polymers. It is a thermoplastic linear aliphatic polyester. It is obtained by the copolymerization of 3-hydroxybutanoic acid and 3-hydroxypentanoic acid. PHBV is used in speciality packaging, orthopedic devices and in controlled release of drugs. PHBV undergoes bacterial degradation in the environment.
Biodegradable additives are additives that enhance the biodegradation of polymers by allowing microorganisms to utilize the carbon within the polymer chain as a source of energy. Biodegradable additives attract microorganisms to the polymer through quorum sensing after biofilm creation on the plastic product. Additives are generally in masterbatch formation that use carrier resins such as polyethylene (PE), polypropylene (PP), polystyrene (PS) or polyethylene terephthalate (PET).

Polybutylene succinate (PBS) is a thermoplastic polymer resin of the polyester family. PBS is a biodegradable aliphatic polyester with properties that are comparable to polypropylene.
Poly(ethylene adipate) or PEA is an aliphatic polyester. It is most commonly synthesized from a polycondensation reaction between ethylene glycol and adipic acid. PEA has been studied as it is biodegradable through a variety of mechanisms and also fairly inexpensive compared to other polymers. Its lower molecular weight compared to many polymers aids in its biodegradability.

Biodegradable athletic footwear is athletic footwear that uses biodegradable materials with the ability to compost at the end-of-life phase. Such materials include natural biodegradable polymers, synthetic biodegradable polymers, and biodegradable blends. The use of biodegradable materials is a long-term solution to landfill pollution that can significantly help protect the natural environment by replacing the synthetic, non-biodegradable polymers found in athletic footwear.

Edible packaging refers to packaging which is edible and biodegradable.
The methods for sequence analysis of synthetic polymers differ from the sequence analysis of biopolymers. Synthetic polymers are produced by chain-growth or step-growth polymerization and show thereby polydispersity, whereas biopolymers are synthesized by complex template-based mechanisms and are sequence-defined and monodisperse. Synthetic polymers are a mixture of macromolecules of different length and sequence and are analysed via statistical measures.



















