Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2.

A photonic crystal is an optical nanostructure in which the refractive index changes periodically. This affects the propagation of light in the same way that the structure of natural crystals gives rise to X-ray diffraction and that the atomic lattices of semiconductors affect their conductivity of electrons. Photonic crystals occur in nature in the form of structural coloration and animal reflectors, and, as artificially produced, promise to be useful in a range of applications.

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.

Cyclopentene is a chemical compound with the formula (CH2)3(CH)2. It is a colorless liquid with a petrol-like odor. It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%. It is one of the principal cycloalkenes.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives. Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.
An inorganic polymer is a polymer with a skeletal structure that does not include carbon atoms in the backbone. Polymers containing inorganic and organic components are sometimes called hybrid polymers, and most so-called inorganic polymers are hybrid polymers. One of the best known examples is polydimethylsiloxane, otherwise known commonly as silicone rubber. Inorganic polymers offer some properties not found in organic materials including low-temperature flexibility, electrical conductivity, and nonflammability. The term inorganic polymer refers generally to one-dimensional polymers, rather than to heavily crosslinked materials such as silicate minerals. Inorganic polymers with tunable or responsive properties are sometimes called smart inorganic polymers. A special class of inorganic polymers are geopolymers, which may be anthropogenic or naturally occurring.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.
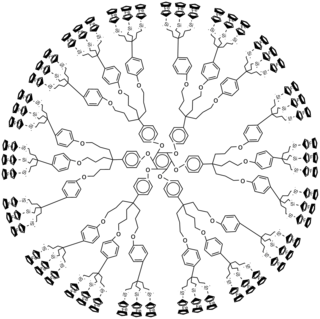
A metallodendrimer is a type of dendrimer with incorporated metal atoms. The development of this type of material is actively pursued in academia.
A high-refractive-index polymer (HRIP) is a polymer that has a refractive index greater than 1.50.
Conjugated microporous polymers (CMPs) are a sub-class of porous materials that are related to structures such as zeolites, metal-organic frameworks, and covalent organic frameworks, but are amorphous in nature, rather than crystalline. CMPs are also a sub-class of conjugated polymers and possess many of the same properties such as conductivity, mechanical rigidity, and insolubility. CMPs are created through the linking of building blocks in a π-conjugated fashion and possess 3-D networks. Conjugation extends through the system of CMPs and lends conductive properties to CMPs. Building blocks of CMPs are attractive in that the blocks possess broad diversity in the π units that can be used and allow for tuning and optimization of the skeleton and subsequently the properties of CMPs. Most building blocks have rigid components such as alkynes that cause the microporosity. CMPs have applications in gas storage, heterogeneous catalysis, light emitting, light harvesting, and electric energy storage.

Smart inorganic polymers (SIPs) are hybrid or fully inorganic polymers with tunable (smart) properties such as stimuli responsive physical properties (shape, conductivity, rheology, bioactivity, self-repair, sensing etc.). While organic polymers are often petrol-based, the backbones of SIPs are made from elements other than carbon which can lessen the burden on scarce non-renewable resources and provide more sustainable alternatives. Common backbones utilized in SIPs include polysiloxanes, polyphosphates, and polyphosphazenes, to name a few.
Diketopyrrolopyrroles (DPPs) are organic dyes and pigments based on the heterocyclic dilactam 2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione, widely used in optoelectronics. DPPs were initially used as pigments in the painting industry due to their high resistance to photodegradation. More recently, DPP derivatives have been also investigated as promising fluorescent dyes for bioimaging applications, as well as components of materials for use in organic electronics.

Timothy M. Swager is an American Scientist and the John D. MacArthur Professor of Chemistry at the Massachusetts Institute of Technology. His research is at the interface of chemistry and materials science, with specific interests in carbon nanomaterials, polymers, and liquid crystals. He is an elected member of the National Academy of Sciences, American Academy of Arts and Sciences, and the National Academy of Inventors.
Brigitte Voit is a German chemist and professor of chemistry. She holds the chair Organic Chemistry of Polymers at the Faculty of Chemistry of the TU Dresden and is head of the Institute of Macromolecular Chemistry at the Leibniz Institute of Polymer Research in Dresden. From September 1, 2002 to July 31, 2022 she was also member of the Board of Management/CSO of the IPF Dresden.

Subi Jacob George is an Indian organic chemist, known for his work in the fields of supramolecular chemistry, materials chemistry and polymer chemistry. His research interests includes organic and supramolecular synthesis, functional organic materials, supramolecular polymers, chiral amplification, and hybrid materials.

Rajendra Rathore was an organic chemist and professor at Marquette University in Milwaukee, Wisconsin as Pfletschinger-Habermann professor of organic chemistry. He made important contributions in the area of supramolecular chemistry, synthesis of novel electro-active molecules, and drug discovery. Rathore died on February 16, 2018, after complications from chronic pulmonary sarcoidosis.

Inverse vulcanization is a process that produces polysulfide polymers, which also contain some organic linkers. In contrast, sulfur vulcanization produces material that is predominantly organic but has a small percentage of polysulfide crosslinks.

Helma B. Wennemers is a German organic chemist. She is a professor of organic chemistry at the Swiss Federal Institute of Technology in Zurich.
Tomislav Friščić holds the Leverhulme International Professorship and Chair in Green and Sustainable chemistry at the University of Birmingham. His research focus is at the interface of green chemistry and materials science, developing solvent-free chemistry and mechanochemistry for the cleaner, efficient synthesis of molecules and materials, including organic solids such as pharmaceutical cocrystals, coordination polymers and Metal-Organic Frameworks (MOFs), and a wide range of organic targets such as active pharmaceutical ingredients. He is a Fellow of the Royal Society of Chemistry (RSC), member of the College of New Scholars, Artists and Scientists of the Royal Society of Canada and a corresponding member of the Croatian Academy of Sciences and Arts. He has served on the Editorial Board of CrystEngComm, the Early Career Board of the ACS journal ACS Sustainable Chemistry & Engineering, and was as an Associate Editor for the journal Molecular Crystals & Liquid Crystals as well as for the journal Synthesis. He was a Topic Editor and Social Media Editor, and is currently a member of the Editorial Advisory Board of the journal Crystal Growth & Design published by the American Chemical Society (ACS). He famously has a dog named Zizi.
Photonic crystal sensors use photonic crystals: nanostructures composed of periodic arrangements of dielectric materials that interact with light depending on their particular structure, reflecting lights of specific wavelengths at specific angles. Any change in the periodicity or refractive index of the structure can give rise to a change in the reflected color, or the color perceived by the observer or a spectrometer. That simple principle makes them useful colorimetric intuitive sensors for different applications including, but not limited to, environmental analysis, temperature sensing, magnetic sensing, biosensing, diagnostics, food quality control, security, and mechanical sensing. Many animals in nature such as fish or beetles employ responsive photonic crystals for camouflage, signaling or to bait their prey. The variety of materials utilizable in such structures ranging from inorganic, organic as well as plasmonic metal nanoparticles makes these structures highly customizable and versatile. In the case of inorganic materials, variation of the refractive index is the most commonly exploited effect in sensing, while periodicity change is more commonly exhibited in polymer-based sensors. Besides their small size, current developments in manufacturing technologies have made them easy and cheap to fabricate on a larger scale, making them mass-producible and practical.













