
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process.
Sodium silicate is a generic name for chemical compounds with the formula Na
2xSi
yO
2y+x or (Na
2O)
x·(SiO
2)
y, such as sodium metasilicate, sodium orthosilicate, and sodium pyrosilicate. The anions are often polymeric. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts.
The Bayer process is the principal industrial means of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer. Bauxite, the most important ore of aluminium, contains only 30–60% aluminium oxide (Al2O3), the rest being a mixture of silica, various iron oxides, and titanium dioxide. The aluminium oxide must be further purified before it can be refined into aluminium.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.

Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.
The first isolation of deoxyribonucleic acid (DNA) was done in 1869 by Friedrich Miescher. DNA extraction is the process of isolating DNA from the cells of an organism isolated from a sample, typically a biological sample such as blood, saliva, or tissue. It involves breaking open the cells, removing proteins and other contaminants, and purifying the DNA so that it is free of other cellular components. The purified DNA can then be used for downstream applications such as PCR, sequencing, or cloning. Currently, it is a routine procedure in molecular biology or forensic analyses.
The lead chamber process was an industrial method used to produce sulfuric acid in large quantities. It has been largely supplanted by the contact process.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers in solution into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol–gel process is used to produce ceramic nanoparticles.
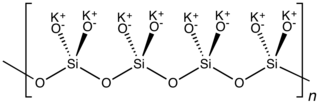
Potassium silicate is the name for a family of inorganic compounds. The most common potassium silicate has the formula K2SiO3, samples of which contain varying amounts of water. These are white solids or colorless solutions.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.
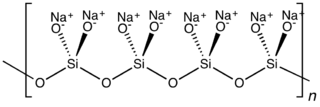
Sodium metasilicate is the chemical substance with formula Na
2SiO
3, which is the main component of commercial sodium silicate solutions. It is an ionic compound consisting of sodium cations Na+
and the polymeric metasilicate anions [–SiO2−
3–]n. It is a colorless crystalline hygroscopic and deliquescent solid, soluble in water but not in alcohols.

Fumed silica, also known as pyrogenic silica because it is produced in a flame, consists of microscopic droplets of amorphous silica fused into branched, chainlike, three-dimensional secondary particles which then agglomerate into tertiary particles. The resulting powder has an extremely low bulk density and high surface area. Its three-dimensional structure results in viscosity-increasing, thixotropic behavior when used as a thickener or reinforcing filler.
In ore deposit geology, supergene processes or enrichment are those that occur relatively near the surface as opposed to deep hypogene processes. Supergene processes include the predominance of meteoric water circulation (i.e. water derived from precipitation) with concomitant oxidation and chemical weathering. The descending meteoric waters oxidize the primary (hypogene) sulfide ore minerals and redistribute the metallic ore elements. Supergene enrichment occurs at the base of the oxidized portion of an ore deposit. Metals that have been leached from the oxidized ore are carried downward by percolating groundwater, and react with hypogene sulfides at the supergene-hypogene boundary. The reaction produces secondary sulfides with metal contents higher than those of the primary ore. This is particularly noted in copper ore deposits where the copper sulfide minerals chalcocite (Cu2S), covellite (CuS), digenite (Cu18S10), and djurleite (Cu31S16) are deposited by the descending surface waters.

The alkali–silica reaction (ASR), also commonly known as concrete cancer, is a deleterious internal swelling reaction that occurs over time in concrete between the highly alkaline cement paste and the reactive amorphous silica found in many common aggregates, given sufficient moisture.

Hydrated silica is a form of silicon dioxide, which has a variable amount of water in the formula. When dissolved in water, it is usually known as silicic acid. It is found in nature as opal, and in the cell walls of diatoms. It is also synthetically manufactured for use in toothpaste as an abrasive to assist in cleaning. Hydrated silica can be dehydrated to produce silica gel, which is used as a desiccant. It is also used in various paints and varnishes and in the production of beer.
Porous glass is glass that includes pores, usually in the nanometre- or micrometre-range, commonly prepared by one of the following processes: through metastable phase separation in borosilicate glasses (such as in their system SiO2-B2O3-Na2O), followed by liquid extraction of one of the formed phases; through the sol-gel process; or simply by sintering glass powder.

Concrete degradation may have many different causes. Concrete is mostly damaged by the corrosion of reinforcement bars due to the carbonatation of hardened cement paste or chloride attack under wet conditions. Chemical damage is caused by the formation of expansive products produced by chemical reactions, by aggressive chemical species present in groundwater and seawater, or by microorganisms Other damaging processes can also involve calcium leaching by water infiltration, physical phenomena initiating cracks formation and propagation, fire or radiant heat, aggregate expansion, sea water effects, leaching, and erosion by fast-flowing water.
Synthetic magnesium silicates are white, odorless, finely divided powders formed by the precipitation reaction of water-soluble sodium silicate and a water-soluble magnesium salt such as magnesium chloride, magnesium nitrate or magnesium sulfate. The composition of the precipitate depends on the ratio of the components in the reaction medium, the addition of the correcting substances, and the way in which they are precipitated.
In chemistry, a silicic acid is any chemical compound containing the element silicon attached to oxide and hydroxyl groups, with the general formula [H2xSiOx+2]n or, equivalently, [SiOx(OH)4−2x]n. Orthosilicic acid is a representative example. Silicic acids are rarely observed in isolation, but are thought to exist in aqueous solutions, including seawater, and play a role in biomineralization. They are typically colorless weak acids that are sparingly soluble in water. Like the silicate anions, which are their better known conjugate bases, silicic acids are proposed to be oligomeric or polymeric. No simple silicic acid has ever been identified, since these species are primarily of theoretical interest.










