A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture. A colloid has a dispersed phase and a continuous phase. The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre.

A micelle or micella is an aggregate of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension. A typical micelle in water forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre.

Nanomaterials describe, in principle, materials of which a single unit is sized between 1 and 100 nm.

Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication.

A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol-gel process is used to produce ceramic nanoparticles.
Nanomaterial-based catalysts are usually heterogeneous catalysts broken up into metal nanoparticles in order to enhance the catalytic process. Metal nanoparticles have high surface area, which can increase catalytic activity. Nanoparticle catalysts can be easily separated and recycled. They are typically used under mild conditions to prevent decomposition of the nanoparticles.
Reversed-phase chromatography includes any chromatographic method that uses a hydrophobic stationary phase. RPC refers to liquid chromatography.
Polymer nanocomposites (PNC) consist of a polymer or copolymer having nanoparticles or nanofillers dispersed in the polymer matrix. These may be of different shape, but at least one dimension must be in the range of 1–50 nm. These PNC's belong to the category of multi-phase systems that consume nearly 95% of plastics production. These systems require controlled mixing/compounding, stabilization of the achieved dispersion, orientation of the dispersed phase, and the compounding strategies for all MPS, including PNC, are similar. Alternatively, polymer can be infiltrated into 1D, 2D, 3D preform creating high content polymer nanocomposites.
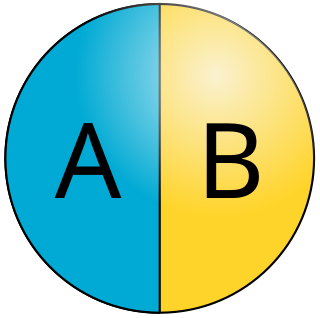
Janus particles are special types of nanoparticles or microparticles whose surfaces have two or more distinct physical properties. This unique surface of Janus particles allows two different types of chemistry to occur on the same particle. The simplest case of a Janus particle is achieved by dividing the particle into two distinct parts, each of them either made of a different material, or bearing different functional groups. For example, a Janus particle may have one-half of its surface composed of hydrophilic groups and the other half hydrophobic groups, the particles might have two surfaces of different color, fluorescence, or magnetic properties. This gives these particles unique properties related to their asymmetric structure and/or functionalization.
An anti-graffiti coating is a coating that prevents graffiti paint from bonding to surfaces.

The Center of Excellence (CoE) in Nanotechnology is located at the Asian Institute of Technology campus. It is one of the eight centers of excellence in Thailand.
Plasma polymerization uses plasma sources to generate a gas discharge that provides energy to activate or fragment gaseous or liquid monomer, often containing a vinyl group, in order to initiate polymerization. Polymers formed from this technique are generally highly branched and highly cross-linked, and adhere to solid surfaces well. The biggest advantage to this process is that polymers can be directly attached to a desired surface while the chains are growing, which reduces steps necessary for other coating processes such as grafting. This is very useful for pinhole-free coatings of 100 picometers to 1-micrometer thickness with solvent insoluble polymers.
The Stöber process is a chemical process used to prepare silica particles of controllable and uniform size for applications in materials science. It was pioneering when it was reported by Werner Stöber and his team in 1968, and remains today the most widely used wet chemistry synthetic approach to silica nanoparticles. It is an example of a sol-gel process wherein a molecular precursor is first reacted with water in an alcoholic solution, the resulting molecules then joining together to build larger structures. The reaction produces silica particles with diameters ranging from 50 to 2000 nm, depending on conditions. The process has been actively researched since its discovery, including efforts to understand its kinetics and mechanism – a particle aggregation model was found to be a better fit for the experimental data than the initially hypothesized LaMer model. The newly acquired understanding has enabled researchers to exert a high degree of control over particle size and distribution and to fine-tune the physical properties of the resulting material in order to suit intended applications.
Silanization of silicon and mica is the coating of these materials with a thin layer of self assembling units.
Liquid–feed flame spray pyrolysis (LF-FSP) is one of the most recent iterations in flame spray pyrolysis (FSP) powder production technology. FSP produces metal oxide powders from highly volatile gaseous metal chlorides that are decomposed/oxidized in hydrogen-oxygen flames to form nano-oxide powders. However, products made from FSP's vapor-phase process are limited to Al-, Ti-, Zr-, and Si-based oxides from their metal chlorides. Thus, interest in producing more complex materials required a new methodology, LF-FSP.
The surface chemistry of paper is responsible for many important paper properties, such as gloss, waterproofing, and printability. Many components are used in the paper-making process that affect the surface.

Nanoparticles are classified as having at least one of its dimensions in the range of 1-100 nanometers (nm). The small size of nanoparticles allows them to have unique characteristics which may not be possible on the macro-scale. Self-assembly is the spontaneous organization of smaller subunits to form larger, well-organized patterns. For nanoparticles, this spontaneous assembly is a consequence of interactions between the particles aimed at achieving a thermodynamic equilibrium and reducing the system’s free energy. The thermodynamics definition of self-assembly was introduced by Professor Nicholas A. Kotov. He describes self-assembly as a process where components of the system acquire non-random spatial distribution with respect to each other and the boundaries of the system. This definition allows one to account for mass and energy fluxes taking place in the self-assembly processes.
1,7-Octadiene (C8H14) is a light flammable organic compound.

Nanosized aluminium oxide occurs in the form of spherical or nearly spherical nanoparticles, and in the form of oriented or undirected fibers.