
The polymerase chain reaction (PCR) is a method widely used to make millions to billions of copies of a specific DNA sample rapidly, allowing scientists to amplify a very small sample of DNA sufficiently to enable detailed study. PCR was invented in 1983 by American biochemist Kary Mullis at Cetus Corporation. Mullis and biochemist Michael Smith, who had developed other essential ways of manipulating DNA, were jointly awarded the Nobel Prize in Chemistry in 1993.

Reverse transcription polymerase chain reaction (RT-PCR) is a laboratory technique combining reverse transcription of RNA into DNA and amplification of specific DNA targets using polymerase chain reaction (PCR). It is primarily used to measure the amount of a specific RNA. This is achieved by monitoring the amplification reaction using fluorescence, a technique called real-time PCR or quantitative PCR (qPCR). Confusion can arise because some authors use the acronym RT-PCR to denote real-time PCR. In this article, RT-PCR will denote Reverse Transcription PCR. Combined RT-PCR and qPCR are routinely used for analysis of gene expression and quantification of viral RNA in research and clinical settings.
The thermal cycler is a laboratory apparatus most commonly used to amplify segments of DNA via the polymerase chain reaction (PCR). Thermal cyclers may also be used in laboratories to facilitate other temperature-sensitive reactions, including restriction enzyme digestion or rapid diagnostics. The device has a thermal block with holes where tubes holding the reaction mixtures can be inserted. The cycler then raises and lowers the temperature of the block in discrete, pre-programmed steps.
Taq polymerase is a thermostable DNA polymerase I named after the thermophilic eubacterial microorganism Thermus aquaticus, from which it was originally isolated by Chinese scientist Alice Chien et al. in 1976. Its name is often abbreviated to Taq or Taq pol. It is frequently used in the polymerase chain reaction (PCR), a method for greatly amplifying the quantity of short segments of DNA.
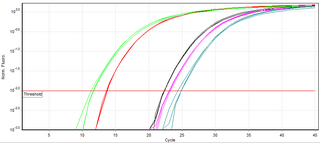
A real-time polymerase chain reaction is a laboratory technique of molecular biology based on the polymerase chain reaction (PCR). It monitors the amplification of a targeted DNA molecule during the PCR, not at its end, as in conventional PCR. Real-time PCR can be used quantitatively and semi-quantitatively.

New England Biolabs (NEB) is an American life sciences company which produces and supplies recombinant and native enzyme reagents for life science research. It also provides products and services supporting genome editing, synthetic biology and next-generation sequencing. NEB also provides free access to research tools such as REBASE, InBASE, and Polbase.
Serum Institute of India (SII) is an Indian multinational biotechnology and biopharmaceuticals company, based in Pune. It is the world's largest manufacturer of vaccines by volume. It was founded by Cyrus Poonawalla in 1966 and is a part of Cyrus Poonawalla Group.
Multiplex polymerase chain reaction refers to the use of polymerase chain reaction to amplify several different DNA sequences simultaneously. This process amplifies DNA in samples using multiple primers and a temperature-mediated DNA polymerase in a thermal cycler. The primer design for all primers pairs has to be optimized so that all primer pairs can work at the same annealing temperature during PCR.
Recombinase polymerase amplification (RPA) is a single tube, isothermal alternative to the polymerase chain reaction (PCR). By adding a reverse transcriptase enzyme to an RPA reaction, it can detect RNA as well as DNA, without the need for a separate step to produce cDNA. Because it is isothermal, RPA can use much simpler equipment than PCR, which requires a thermal cycler. Operating best at temperatures of 37–42 °C and still working, albeit more slowly, at room temperature means RPA reactions can in theory be run quickly by simply holding a tube in the hand. This makes RPA an excellent candidate for developing low-cost, rapid, point-of-care molecular tests. An international quality assessment of molecular detection of Rift Valley fever virus performed as well as the best RT-PCR tests, detecting less concentrated samples missed by some PCR tests and an RT-LAMP test. RPA was developed and launched by TwistDx Ltd., a biotechnology company based in Cambridge, UK.

Cepheid is an American molecular diagnostics company that is a wholly owned subsidiary of Danaher Corporation. Its systems automate traditional nucleic acid tests. The tests can be used to identify and analyze pathogens and genetic disorders. Cepheid sells clinical tests for healthcare-associated infections, infectious diseases, sexual health, oncology and genetics.
Tom Brown FRSC FRSE is a British chemist, biotechnologist, and entrepreneur. He is the Professor of Nucleic acid chemistry at the Department of Chemistry and Department of Oncology at the University of Oxford. Currently, he is serving as the President of the Chemical Biology Interface Division of the Royal Society of Chemistry. He is best known for his contribution in the field of DNA Repair, DNA Click chemistry, and in the application of Molecular genetics in forensics and diagnostics.

The Philippine Genome Center (PGC) is a multi-disciplinary research facility in Quezon City, Metro Manila, Philippines which specializes in genomics.

Novacyt Group is an Anglo-French biotechnology group focused on clinical diagnostics, with offices in Camberley, Surrey, United Kingdom and Vélizy-Villacoublay, France. The company produces in vitro and molecular diagnostic tests, supplying assays and reagents worldwide. Its business units include Primerdesign, Microgen Bioproducts and Lab21 Healthcare.

Dame Sarah Catherine Gilbert FRS is an English vaccinologist who is a Professor of Vaccinology at the University of Oxford and co-founder of Vaccitech. She specialises in the development of vaccines against influenza and emerging viral pathogens. She led the development and testing of the universal flu vaccine, which underwent clinical trials in 2011.

COVID-19 testing involves analyzing samples to assess the current or past presence of SARS-CoV-2, the virus that cases COVID-19 and is responsible for the COVID-19 pandemic. The two main types of tests detect either the presence of the virus or antibodies produced in response to infection. Molecular tests for viral presence through its molecular components are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks. Antibody tests instead show whether someone once had the disease. They are less useful for diagnosing current infections because antibodies may not develop for weeks after infection. It is used to assess disease prevalence, which aids the estimation of the infection fatality rate.
Catherine Mary Green is an English biologist who is an Associate Professor in Chromosome Dynamics at the Wellcome Centre for Human Genetics at the University of Oxford. Her research considers chromosome stability during the replication of DNA. During the COVID-19 pandemic Green was part of the Oxford team who developed the Oxford–AstraZeneca COVID-19 vaccine.
Truenat is a chip-based, point-of-care, rapid molecular test for diagnosis of infectious diseases. The technology is based on the Taqman RTPCR chemistry which can be performed on the portable, battery operated Truelab Real Time micro PCR platform. Truenat is developed and manufactured by Goa-based Molbio Diagnostics Private Limited.
Seegene, Inc is a Korean manufacturer of in vitro diagnostic (IVD) products, particularly molecular diagnostics. Its portfolio includes a range of assays and screening products for sepsis, respiratory diseases such as influenza and respiratory syncytial virus, as well as sexually transmitted infections (STIs). It was founded in 2000. In early 2020, it began developing and distributing a range of tests for SARS-CoV-2, the virus that causes COVID-19.

The United Kingdom's response to the COVID-19 pandemic consists of various measures by the healthcare community, the British and devolved governments, the military and the research sector.










