Related Research Articles

A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.
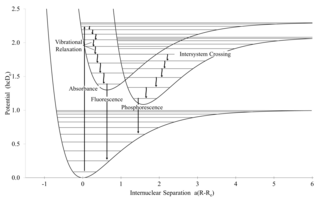
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.
In molecular physics, crystal field theory (CFT) describes the breaking of degeneracies of electron orbital states, usually d or f orbitals, due to a static electric field produced by a surrounding charge distribution. This theory has been used to describe various spectroscopies of transition metal coordination complexes, in particular optical spectra (colors). CFT successfully accounts for some magnetic properties, colors, hydration enthalpies, and spinel structures of transition metal complexes, but it does not attempt to describe bonding. CFT was developed by physicists Hans Bethe and John Hasbrouck van Vleck in the 1930s. CFT was subsequently combined with molecular orbital theory to form the more realistic and complex ligand field theory (LFT), which delivers insight into the process of chemical bonding in transition metal complexes. CFT can be complicated further by breaking assumptions made of relative metal and ligand orbital energies, requiring the use of inverted ligand field theory (ILFT) to better describe bonding.
Ligand field theory (LFT) describes the bonding, orbital arrangement, and other characteristics of coordination complexes. It represents an application of molecular orbital theory to transition metal complexes. A transition metal ion has nine valence atomic orbitals - consisting of five nd, one (n+1)s, and three (n+1)p orbitals. These orbitals have the appropriate energy to form bonding interactions with ligands. The LFT analysis is highly dependent on the geometry of the complex, but most explanations begin by describing octahedral complexes, where six ligands coordinate with the metal. Other complexes can be described with reference to crystal field theory. Inverted ligand field theory (ILFT) elaborates on LFT by breaking assumptions made about relative metal and ligand orbital energies.
A spectrochemical series is a list of ligands ordered by ligand "strength", and a list of metal ions based on oxidation number, group and element. For a metal ion, the ligands modify the difference in energy Δ between the d orbitals, called the ligand-field splitting parameter in ligand field theory, or the crystal-field splitting parameter in crystal field theory. The splitting parameter is reflected in the ion's electronic and magnetic properties such as its spin state, and optical properties such as its color and absorption spectrum.
The 18-electron rule is a chemical rule of thumb used primarily for predicting and rationalizing formulas for stable transition metal complexes, especially organometallic compounds. The rule is based on the fact that the valence orbitals in the electron configuration of transition metals consist of five (n−1)d orbitals, one ns orbital, and three np orbitals, where n is the principal quantum number. These orbitals can collectively accommodate 18 electrons as either bonding or non-bonding electron pairs. This means that the combination of these nine atomic orbitals with ligand orbitals creates nine molecular orbitals that are either metal-ligand bonding or non-bonding. When a metal complex has 18 valence electrons, it is said to have achieved the same electron configuration as the noble gas in the period, lending stability to the complex. Transition metal complexes that deviate from the rule are often interesting or useful because they tend to be more reactive. The rule is not helpful for complexes of metals that are not transition metals. The rule was first proposed by American chemist Irving Langmuir in 1921.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.

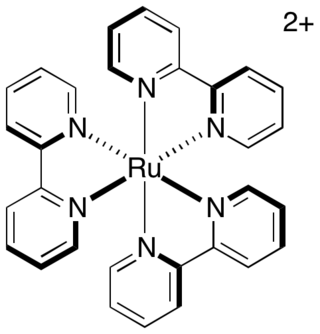
Tris(bipyridine)ruthenium(II) chloride is the chloride salt coordination complex with the formula [Ru(bpy)3]Cl2. This polypyridine complex is a red crystalline salt obtained as the hexahydrate, although all of the properties of interest are in the cation [Ru(bpy)3]2+, which has received much attention because of its distinctive optical properties. The chlorides can be replaced with other anions, such as PF6−.
The d electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination complexes; crystal field theory and ligand field theory, which is a more advanced version based on molecular orbital theory. However the d electron count of an atom in a complex is often different from the d electron count of a free atom or a free ion of the same element.
Spin states when describing transition metal coordination complexes refers to the potential spin configurations of the central metal's d electrons. For several oxidation states, metals can adopt high-spin and low-spin configurations. The ambiguity only applies to first row metals, because second- and third-row metals are invariably low-spin. These configurations can be understood through the two major models used to describe coordination complexes; crystal field theory and ligand field theory.
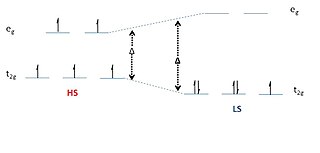
Spin crossover (SCO) is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to an external stimulus. The stimuli can include temperature or pressure. Spin crossover is sometimes referred to as spin transition or spin equilibrium behavior. The change in spin state usually involves interchange of low spin (LS) and high spin (HS) configuration.

Metal L-edge spectroscopy is a spectroscopic technique used to study the electronic structures of transition metal atoms and complexes. This method measures X-ray absorption caused by the excitation of a metal 2p electron to unfilled d orbitals, which creates a characteristic absorption peak called the L-edge. Similar features can also be studied by Electron Energy Loss Spectroscopy. According to the selection rules, the transition is formally electric-dipole allowed, which not only makes it more intense than an electric-dipole forbidden metal K pre-edge transition, but also makes it more feature-rich as the lower required energy results in a higher-resolution experiment.
Photochemical reduction of carbon dioxide harnesses solar energy to convert CO2 into higher-energy products. Environmental interest in producing artificial systems is motivated by recognition that CO2 is a greenhouse gas. The process has not been commercialized.
Methylene is an organic compound with the chemical formula CH
2. It is a colourless gas that fluoresces in the mid-infrared range, and only persists in dilution, or as an adduct.
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry [M(H2O)n]z+. Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand, but of course many complexes are known to consist of a mix of aquo and other ligands.

Charge-transfer bands are a characteristic feature of the optical spectra of many compounds. These bands are typically more intense than d–d transitions. They typically exhibit solvatochromism, consistent with shifts of electron density that would be sensitive to solvation.
Photoredox catalysis is a branch of photochemistry that uses single-electron transfer. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes, and semiconductors. While organic photoredox catalysts were dominant throughout the 1990s and early 2000s, soluble transition-metal complexes are more commonly used today.
Kainosymmetry describes the first atomic orbital of each azimuthal quantum number (ℓ). Such orbitals include 1s, 2p, 3d, 4f, 5g, and so on. The term kainosymmetric was coined by Sergey Shchukarev. Pekka Pyykkö referred to such orbitals as primogenic instead. Such orbitals are much smaller than all other orbitals with the same ℓ and have no radial nodes, giving the elements that fill them special properties. They are usually less metallic than their heavier homologues, prefer lower oxidation states, and have smaller atomic and ionic radii.
Transition metal complexes of 2,2'-bipyridine are coordination complexes containing one or more 2,2'-bipyridine ligands. Complexes have been described for all of the transition metals. Although few have any practical value, these complexes have been influential. 2,2'-Bipyridine (bipy) is classified as a diimine ligand. Unlike the structures of pyridine complexes, the two rings in bipy are coplanar, which facilitates electron delocalization. As a consequence of this delocalization, bipy complexes often exhibit distinctive optical and redox properties.

Tris(bipyridine)iron(II) chloride is the chloride salt of the coordination complex tris(bipyridine)iron(II), [Fe(C10H8N2)3]2+. It is a red solid. In contrast to tris(bipyridine)ruthenium(II), this iron complex is not a useful photosensitizer because its excited states relax too rapidly, a consequence of the primogenic effect.
References
- ↑ McCusker, James K. (2019). "Electronic structure in the transition metal block and its implications for light harvesting". Science. 363 (6426): 484–488. Bibcode:2019Sci...363..484M. doi: 10.1126/science.aav9104 . PMID 30705184. S2CID 59565142.
- ↑ M. Kaupp (2007). "The Role of Radial Nodes of Atomic Orbitals for Chemical Bonding and the Periodic Table". J. Comput. Chem. 28 (1): 320–325. doi:10.1002/jcc.20522. PMID 17143872.
- ↑ Pyykko, Pekka (1988). "Relativistic effects in structural chemistry". Chemical Reviews. 88 (3): 563–594. doi:10.1021/cr00085a006.