A membrane transport protein is a membrane protein involved in the movement of ions, small molecules, or macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion or active transport. The two main types of proteins involved in such transport are broadly categorized as either channels or carriers. The solute carriers and atypical SLCs are secondary active or facilitative transporters in humans.

Escherichia is a genus of Gram-negative, non-spore-forming, facultatively anaerobic, rod-shaped bacteria from the family Enterobacteriaceae. In those species which are inhabitants of the gastrointestinal tracts of warm-blooded animals, Escherichia species provide a portion of the microbially derived vitamin K for their host. A number of the species of Escherichia are pathogenic. The genus is named after Theodor Escherich, the discoverer of Escherichia coli. Physiologically, it is a facultative aerobe, meaning that it can grow happily with or without oxygen, but it cannot grow at extremes of temperature or pH nor can it degrade dangerous pollutants, photosynthesize, or do a variety of other things that interest microbiologists.
Osmotic shock or osmotic stress is physiologic dysfunction caused by a sudden change in the solute concentration around a cell, which causes a rapid change in the movement of water across its cell membrane. Under conditions of high concentrations of either salts, substrates or any solute in the supernatant, water is drawn out of the cells through osmosis. This also inhibits the transport of substrates and cofactors into the cell thus “shocking” the cell. Alternatively, at low concentrations of solutes, water enters the cell in large amounts, causing it to swell and either burst or undergo apoptosis.

Fosfomycin is a broad-spectrum antibiotic produced by certain Streptomyces species, although it can now be made by chemical synthesis.
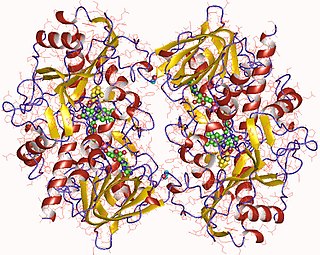
In enzymology, a choline oxidase (EC 1.1.3.17) is an enzyme that catalyzes the chemical reaction
In enzymology, an ectoine synthase (EC 4.2.1.108) is an enzyme that catalyzes the chemical reaction
In enzymology, a diaminobutyrate acetyltransferase (EC 2.3.1.178) is an enzyme that catalyzes the chemical reaction
In enzymology, a diaminobutyrate-2-oxoglutarate transaminase is an enzyme that catalyzes the chemical reaction
A neurotransmitter sodium symporter (NSS) (TC# 2.A.22) is type of neurotransmitter transporter that catalyzes the uptake of a variety of neurotransmitters, amino acids, osmolytes and related nitrogenous substances by a solute:Na+ symport mechanism. The NSS family is a member of the APC superfamily. Its constituents have been found in bacteria, archaea and eukaryotes.
Osmoregulation is the passive regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration of electrolytes to keep the fluids from becoming too diluted or concentrated. Osmotic pressure is a measure of the tendency of water to move into one solution from another by osmosis. The higher the osmotic pressure of a solution, the more water tends to move into it. Pressure must be exerted on the hypertonic side of a selectively permeable membrane to prevent diffusion of water by osmosis from the side containing pure water.
Osmoprotectants or compatible solutes are small organic molecules with neutral charge and low toxicity at high concentrations that act as osmolytes and help organisms survive extreme osmotic stress. Osmoprotectants can be placed in three chemical classes: betaines and associated molecules, sugars and polyols, and amino acids.These molecules accumulate in cells and balance the osmotic difference between the cell's surroundings and the cytosol. In plants, their accumulation can increase survival during stresses such as drought. In extreme cases, such as in bdelloid rotifers, tardigrades, brine shrimp, and nematodes, these molecules can allow cells to survive being completely dried out and let them enter a state of suspended animation called cryptobiosis.

Solute carrier family 6, member 18 also known as SLC6A18 is a protein which in humans is encoded by the SLC6A18 gene.
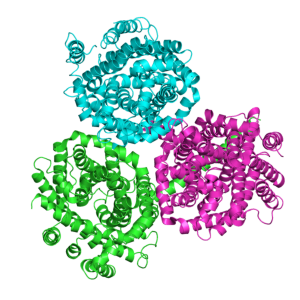
Proteins of the Betaine/Carnitine/Choline Transporter (BCCT) family are found in Gram-negative and Gram-positive bacteria and archaea. The BCCT family is a member a large group of secondary transporters, the APC superfamily. Their common functional feature is that they all transport molecules with a quaternary ammonium group [R-N (CH3)3]. The BCCT family proteins vary in length between 481 and 706 amino acyl residues and possess 12 putative transmembrane α-helical spanners (TMSs). The x-ray structures reveal two 5 TMS repeats with the total number of TMSs being 10. These porters catalyze bidirectional uniport or are energized by pmf-driven or smf-driven proton or sodium ion symport, respectively, or else by substrate:substrate antiport. Some of these permeases exhibit osmosensory and osmoregulatory properties inherent to their polypeptide chains.

Members of the Solute:Sodium Symporter (SSS) Family (TC# 2.A.21) catalyze solute:Na+ symport. The SSS family is within the APC Superfamily. The solutes transported may be sugars, amino acids, organo cations such as choline, nucleosides, inositols, vitamins, urea or anions, depending on the system. Members of the SSS family have been identified in bacteria, archaea and eukaryotes. Almost all functionally well-characterized members normally catalyze solute uptake via Na+ symport.

Escherichia coli ( Anglicized to ; commonly abbreviated E. coli) is a gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms (endotherms). Most E. coli strains are harmless, but some serotypes are pathogenic and can cause serious food poisoning in humans, and are occasionally responsible for product recalls. E. coli are also responsible for a majority of cases of urinary tract infections. The harmless strains are part of the normal flora of the gut, and can benefit their hosts by producing vitamin K2, and by preventing the establishment of pathogenic bacteria within the intestine.

Ammonia transporters are structurally related membrane transport proteins called Amt proteins in bacteria and plants, methylammonium/ammonium permeases (MEPs) in yeast, or Rhesus (Rh) proteins in chordates. In humans, the RhAG, RhBG, and RhCG Rhesus proteins constitute solute carrier family 42 whilst RhD and RhCE form the Rh blood group system. The three-dimensional structure of the ammonia transport protein AmtB from Escherichia coli has been determined by x-ray crystallography revealing a hydrophobic ammonia channel. The human RhCG ammonia transporter was found to have a similar ammonia-conducting channel structure. It was proposed that the erythrocyte Rh complex is a heterotrimer of RhAG, RhD, and RhCE subunits in which RhD and RhCE might play roles in anchoring the ammonia-conducting RhAG subunit to the cytoskeleton. Based on reconstitution experiments, purified RhCG subunits alone can function to transport ammonia. RhCG is required for normal acid excretion by the mouse kidney and epididymis.

OmpT is an aspartyl protease found on the outer membrane of Escherichia coli. OmpT is a subtype of the family of omptin proteases, which are found on some gram-negative species of bacteria.
The p-aminobenzoyl-glutamate transporter(AbgT) family is a family of transporter proteins belonging to the ion transporter (IT) superfamily. The AbgT family consists of the AbgT protein of E. coli and the MtrF drug exporter of Neisseria gonorrhoeae. The former protein is apparently cryptic in wild-type cells, but when expressed on a high copy number plasmid, or when expressed at higher levels due to mutation, it appeared to allow uptake and subsequent utilization of p-aminobenzoyl-glutamate as a source of p-aminobenzoate for p-aminobenzoate auxotrophs. p-Aminobenzoate is a constituent of and a precursor for the biosynthesis of folic acid. MtrF was annotated as a putative drug efflux pump.
P fimbriae or P pili or Pap are chaperon-usher type fimbrial appendages found on the surface of many Escherichia coli bacteria. The P fimbriae is considered to be one of the most important virulence factor in uropathogenic E. coli and plays an important role in upper urinary tract infections. P fimbriae mediate adherence to host cells, a key event in the pathogenesis of urinary tract infections.

Sodium- and chloride-dependent betaine transporter, also known as Na(+)/Cl(-) betaine/GABA transporter (BGT-1), is a protein that in humans is encoded by the SLC6A12 gene. BGT-1 is predominantly expressed in the liver (hepatocytes). It is also expressed in the kidney where it is regulated by NFAT5 during a response to osmotic stress. Further, BGT1 is also present in the leptomeninges surrounding the brain. Deletion of the BGT1 gene in mice did not appear to have any impact on the tendency to develop epilepsy. This is to be expected considering that BGT1 is expressed at far lower levels than GAT1 and also has lower affinity for GABA. This implies that it is not likely to contribute significantly to the inactivation of the inhibitory neurotransmitter GABA.











