
Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid, a type of phenolic acid, found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. The chemical formula of gallic acid is C6H2(OH)3COOH. It is found both free and as part of hydrolyzable tannins. The gallic acid groups are usually bonded to form dimers such as ellagic acid. Hydrolyzable tannins break down on hydrolysis to give gallic acid and glucose or ellagic acid and glucose, known as gallotannins and ellagitannins, respectively.
11β-Hydroxysteroid dehydrogenase type 1, also known as cortisone reductase, is an NADPH-dependent enzyme highly expressed in key metabolic tissues including liver, adipose tissue, and the central nervous system. In these tissues, HSD11B1 reduces cortisone to the active hormone cortisol that activates glucocorticoid receptors. It belongs to the family of short-chain dehydrogenases.

Theaflavin (TF) and its derivatives, known collectively as theaflavins, are antioxidant polyphenols that are formed from the condensation of flavan-3-ols in tea leaves during the enzymatic oxidation of black tea. Theaflavin-3-gallate, theaflavin-3'-gallate, and theaflavin-3-3'-digallate are the main theaflavins. Theaflavins are types of thearubigins, and are therefore reddish in color. Epigallocatechin gallate (EGCG) will metabolize into some theaflavins in the liver. Those molecules contain a tropolone moiety.

Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is the ester of epigallocatechin and gallic acid, and is a type of catechin.

Gallocatechol or gallocatechin (GC) is a flavan-3-ol, a type of chemical compound including catechin, with the gallate residue being in an isomeric trans position. It is one of the antioxidant chemicals found in food.

Cistus salviifolius, common names sage-leaved rock-rose, salvia cistus or Gallipoli rose, is a perennial ligneous plant of the family Cistaceae.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.
Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.
The molecular formula C22H18O11 (molar mass : 458.37 g/mol, exact mass : 458.084911) may refer to:

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.
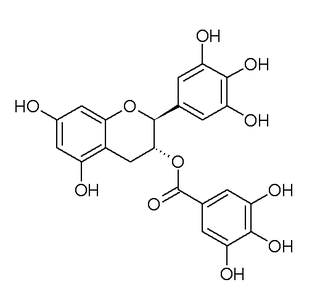
Gallocatechin gallate (GCG) is the ester of gallocatechin and gallic acid and a type of catechin. It is an epimer of epigallocatechin gallate (EGCG).

Glucogallin is chemical compound formed from gallic acid and β-D-glucose. It can be found in oaks species like the North American white oak, European red oak and Amla fruit.

The phenolic content in tea refers to the phenols and polyphenols, natural plant compounds which are found in tea. These chemical compounds affect the flavor and mouthfeel and are speculated to provide potential health benefits. Polyphenols in tea include catechins, theaflavins, tannins, and flavonoids.

Terminalia myriocarpa, the East Indian almond, is a tree species in the genus Terminalia found in Southeast Asia.
Propelargonidins are a type of condensed tannins formed from epiafzelechin. They yield pelargonidin when depolymerized under oxidative conditions.
The molecular formula C30H26O13 (molar mass: 594.52 g/mol, exact mass: 594.137340 u) may refer to:
















