
Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. [1] [2] [3] It yields delphinidin during depolymerisation under oxidative conditions.

Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. [1] [2] [3] It yields delphinidin during depolymerisation under oxidative conditions.
Prodelphinidins are one of the two sorts of tannins in grape (the other being procyanidins) being produced especially in the skin of the berry. [4]
Prodelphinidins can be found in Cistus salviifolius . [5] Gallocatechin-(4→8)-catechin (prodelphinidin B3), gallocatechin-(4→8)-gallocatechin and catechin-(4→8)-gallocatechin can be found in the pomegranate peels. [6] Prodelphinidin B-2 3'-O-gallate can be found in green tea leaves [7] and prodelphinidin B-2 3,3'-di-O-gallate can be found in Myrica rubra . [8]
Prodelphinidin B3 (gallocatechin-(4α→8)-catechin) and prodelphinidin B9 (epigallocatechin-(4α→8)-catechin) can be isolated in beer. [9] [10] Prodelphinidin C2 (gallocatechin-(4α→8)-gallocatechin-(4α→8)-catechin) can be isolated in malt. [11]
The A-type proanthocyanidin epigallocatechin-(2β→7,4β→8)-epicatechin can be found in the leaves of Dioclea lasiophylla , [12]

Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

Dioclea is a genus of flowering plants in the pea family, Fabaceae, that is native to the Americas. The seeds of these legumes are buoyant drift seeds, and are dispersed by rivers.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.
Thearubigins are polymeric polyphenols that are formed during the enzymatic oxidation and condensation of two gallocatechins with the participation of polyphenol oxidases during the fermentation reactions in black tea. Thearubigins are red in colour and are responsible for much of the staining effect of tea. Therefore, a black tea often appears red while a green or white tea has a much clearer appearance. The colour of a black tea, however, is affected by many other factors as well, such as the amount of theaflavins, another oxidized form of polyphenols.

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.

Gallocatechol or gallocatechin (GC) is a flavan-3-ol, a type of chemical compound including catechin, with the gallate residue being in an isomeric trans position.

Cistus salviifolius, common names sage-leaved rock-rose, salvia cistus or Gallipoli rose, is a shrub of the family Cistaceae.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Prodelphinidin B3 is a prodelphinidin dimer found in food products such as barley and beer, in fruits and pod vegetables. It can also be found in pomegranate peels.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.
A type proanthocyanidins are a specific type of proanthocyanidins, which are a class of flavonoid. Proanthocyanidins fall under a wide range of names in the nutritional and scientific vernacular, including oligomeric proanthocyanidins, flavonoids, polyphenols, condensed tannins, and OPCs. Proanthocyanidins were first popularized by French scientist Jacques Masquelier.
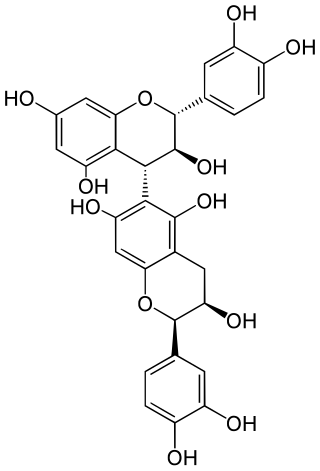
Procyanidin B8 is a B type proanthocyanidin.
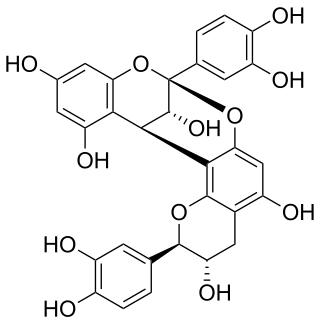
Procyanidin A1 is an A type proanthocyanidin dimer.

Procyanidin A2 is an A type proanthocyanidin.

Afzelechin is a flavan-3-ol, a type of flavonoid. It can be found in Bergenia ligulata. It exists as at least 2 major epimers.

Epicatechin gallate (ECG) is a flavan-3-ol, a type of flavonoid, present in green tea. It is also reported in buckwheat and in grape.
Prorobinetidins are a type of condensed tannins formed from robinetinidol. They form robinetinidin when depolymerized under oxidative conditions.

Stryphnodendron adstringens is a species of legume in the genus Stryphnodendron found in Brazil.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.
Propelargonidins are a type of condensed tannins formed from epiafzelechin. They yield pelargonidin when depolymerized under oxidative conditions.