Related Research Articles

Buckwheat, or common buckwheat, is a flowering plant in the knotweed family Polygonaceae cultivated for its grain-like seeds and as a cover crop. The name "buckwheat" is used for several other species, such as Fagopyrum tataricum, a domesticated food plant raised in Asia.

Carpobrotus edulis is a ground-creeping plant with succulent leaves in the genus Carpobrotus, native to South Africa. Its common names include hottentot-fig, sour fig, ice plant or highway ice plant.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.

Aglaomorpha fortunei, commonly known as gu-sui-bu, is a species of basket fern of the family Polypodiaceae. The plant is native to Eastern Asia, including eastern China.

2-Nonenal is an unsaturated aldehyde. The colorless liquid is an important aroma component of aged beer and buckwheat.

Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.

Procyanidin B2 is a B type proanthocyanidin. Its structure is (−)-Epicatechin-(4β→8)-(−)-epicatechin.

Procyanidin B1 is a procyanidin dimer.

Procyanidin B5 is a B type proanthocyanidin.
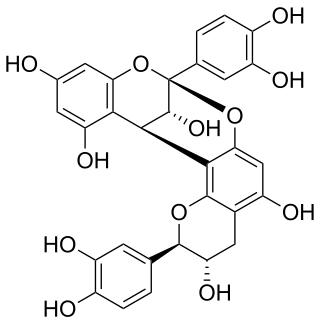
Procyanidin A1 is an A type proanthocyanidin dimer.

Afzelechin is a flavan-3-ol, a type of flavonoid. It can be found in Bergenia ligulata. It exists as at least 2 major epimers.

Selliguea feei is a fern belonging to the genus Selliguea in the family Polypodiaceae. This fern can be collected in Indonesia. The species name feei commemorates the botanist Antoine Laurent Apollinaire Fée.

Grandinin is an ellagitannin. It can be found in Melaleuca quinquenervia leaves and in oaks species like the North American white oak and European red oak. It shows antioxydant activity. It is an astringent compound. It is also found in wine, red or white, aged in oak barrels.

Pteridium esculentum, commonly known as bracken fern, Austral bracken or simply bracken, is a species of the bracken genus native to a number of countries in the Southern Hemisphere. Esculentum means edible. First described as Pteris esculenta by German botanist Georg Forster in 1786, it gained its current binomial name in 1908. The Eora people of the Sydney region knew it as gurgi.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.

Aglaomorpha is a genus of ferns in the subfamily Drynarioideae of the family Polypodiaceae. The Pteridophyte Phylogeny Group classification of 2016 uses this genus name, while other sources use Drynaria to include Aglaomorpha. Species are commonly known as basket ferns. As circumscribed in PPG I, the genus contains around 50 species.

Catechin-7-O-glucoside is a flavan-3-ol glycoside formed from catechin.
Heisteria pallida is a plant species in the genus Heisteria found in Brazil.
References
- ↑ Proliferative effects of flavan-3-ols and propelargonidins from rhizomes of Drynaria fortunei on MCF-7 and osteoblastic cells. Eun Ju Chang, Won Jung Lee, Sung Hee Cho and Sang Won Choi, Archives of Pharmacal Research, August 2003, Volume 26, Issue 8, pages 620–630, doi : 10.1007/BF02976711
- ↑ Identification of galloylated propelargonidins and procyanidins in buckwheat grain and quantification of rutin and flavanols from homostylous hybrids originating from F. esculentum × F. homotropicum. Carolin Ölschläger, Ionela Regos, Friedrich J. Zeller and Dieter Treutter, doi : 10.1016/j.phytochem.2008.01.001
- ↑ LC/ESI-MS/MS characterisation of procyanidins and propelargonidins responsible for the strong antioxidant activity of the edible halophyte Mesembryanthemum edule L. Hanen Falleh, Samia Oueslati, Sylvain Guyot, Alia Ben Dali, Christian Magné, Chedly Abdelly and Riadh Ksouri, doi : 10.1016/j.foodchem.2011.02.049
- ↑ PubChem. "Propelargonidin". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-06-26.
- ↑ A trimeric propelargonidin from stem bark of Heisteria pallida. Verena Dirsch, András Neszmélyia and Hildebert Wagner, Phytochemistry, 3 August 1993, Volume 34, Issue 1, Pages 291–293, doi : 10.1016/S0031-9422(00)90822-7