
Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.
Guaiacol is an organic compound with the formula C6H4(OH)(OCH3). It is a phenolic compound containing a methoxy functional group. Guaiacol appears as a viscous colorless oil, although aged or impure samples are often yellowish. It occurs widely in nature and is a common product of the pyrolysis of wood.

Sinapinic acid, or sinapic acid (Sinapine - Origin: L. Sinapi, sinapis, mustard, Gr., cf. F. Sinapine.), is a small naturally occurring hydroxycinnamic acid. It is a member of the phenylpropanoid family. It is a commonly used matrix in MALDI mass spectrometry. It is a useful matrix for a wide variety of peptides and proteins. It serves well as a matrix for MALDI due to its ability to absorb laser radiation and to also donate protons (H+) to the analyte of interest.

p-Coumaric acid is an organic compound with the formula HOC6H4CH=CHCO2H. It is one of the three isomers of hydroxycinnamic acid. It is a white solid that is only slightly soluble in water but very soluble in ethanol and diethyl ether.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

A polyphenol antioxidant is a hypothetized type of antioxidant, in which each instance would contain a polyphenolic substructure; such instances which have been studied in vitro. Numbering over 4,000 distinct chemical structures, such polyphenols may have antioxidant activity {{{1}}} in vitro (although they are unlikely to be antioxidants in vivo). Hypothetically, they may affect cell-to-cell signaling, receptor sensitivity, inflammatory enzyme activity or gene regulation, although high-quality clinical research has not confirmed any of these possible effects in humans as of 2020.
Thearubigins are polymeric polyphenols that are formed during the enzymatic oxidation and condensation of two gallocatechins with the participation of polyphenol oxidases during the fermentation reactions in black tea. Thearubigins are red in colour and are responsible for much of the staining effect of tea. Therefore, a black tea often appears red while a green or white tea has a much clearer appearance. The colour of a black tea, however, is affected by many other factors as well, such as the amount of theaflavins, another oxidized form of polyphenols.

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.
A type proanthocyanidins are a specific type of proanthocyanidins, which are a class of flavonoid. Proanthocyanidins fall under a wide range of names in the nutritional and scientific vernacular, including oligomeric proanthocyanidins, flavonoids, polyphenols, condensed tannins, and OPCs. Proanthocyanidins were first popularized by French scientist Jacques Masquelier.
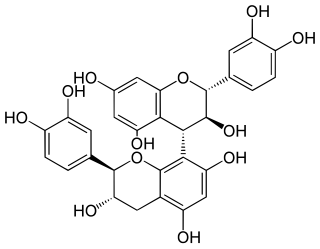
Procyanidin B3 is a B type proanthocyanidin. Procyanidin B3 is a catechin dimer.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

The grape reaction product is a phenolic compound explaining the disappearance of caftaric acid from grape must during processing. It is also found in aged red wines. Its enzymatic production by polyphenol oxidase is important in limiting the browning of musts, especially in white wine production. The product can be recreated in model solutions.

Catechin-7-O-glucoside is a flavan-3-ol glycoside formed from catechin.
















