Related Research Articles

The visual cortex of the brain is the area of the cerebral cortex that processes visual information. It is located in the occipital lobe. Sensory input originating from the eyes travels through the lateral geniculate nucleus in the thalamus and then reaches the visual cortex. The area of the visual cortex that receives the sensory input from the lateral geniculate nucleus is the primary visual cortex, also known as visual area 1 (V1), Brodmann area 17, or the striate cortex. The extrastriate areas consist of visual areas 2, 3, 4, and 5.
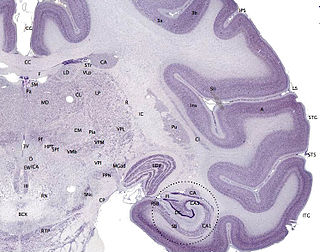
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex, with just 10% consisting of the allocortex. It is separated into two cortices, by the longitudinal fissure that divides the cerebrum into the left and right cerebral hemispheres. The two hemispheres are joined beneath the cortex by the corpus callosum. The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is part of the brain responsible for cognition.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.

The neocortex, also called the neopallium, isocortex, or the six-layered cortex, is a set of layers of the mammalian cerebral cortex involved in higher-order brain functions such as sensory perception, cognition, generation of motor commands, spatial reasoning and language. The neocortex is further subdivided into the true isocortex and the proisocortex.
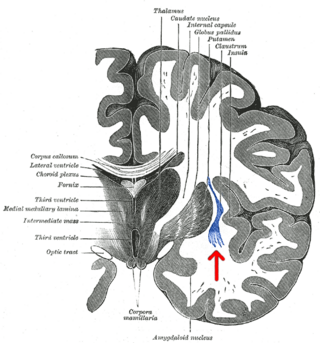
The claustrum is a thin sheet of neurons and supporting glial cells, that connects to the cerebral cortex and subcortical regions including the amygdala, hippocampus and thalamus of the brain. It is located between the insular cortex laterally and the putamen medially, encased by the extreme and external capsules respectively. Blood to the claustrum is supplied by the middle cerebral artery. It is considered to be the most densely connected structure in the brain, and thus hypothesized to allow for the integration of various cortical inputs such as vision, sound and touch, into one experience. Other hypotheses suggest that the claustrum plays a role in salience processing, to direct attention towards the most behaviorally relevant stimuli amongst the background noise. The claustrum is difficult to study given the limited number of individuals with claustral lesions and the poor resolution of neuroimaging.

A cortical column is a group of neurons forming a cylindrical structure through the cerebral cortex of the brain perpendicular to the cortical surface. The structure was first identified by Mountcastle in 1957. He later identified minicolumns as the basic units of the neocortex which were arranged into columns. Each contains the same types of neurons, connectivity, and firing properties. Columns are also called hypercolumn, macrocolumn, functional column or sometimes cortical module. Neurons within a minicolumn (microcolumn) encode similar features, whereas a hypercolumn "denotes a unit containing a full set of values for any given set of receptive field parameters". A cortical module is defined as either synonymous with a hypercolumn (Mountcastle) or as a tissue block of multiple overlapping hypercolumns.

Retinotopy is the mapping of visual input from the retina to neurons, particularly those neurons within the visual stream. For clarity, 'retinotopy' can be replaced with 'retinal mapping', and 'retinotopic' with 'retinally mapped'.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

Pasko Rakic is a Yugoslav-born American neuroscientist, who presently works in the Yale School of Medicine Department of Neuroscience in New Haven, Connecticut. His main research interest is in the development and evolution of the human brain. He was the founder and served as Chairman of the Department of Neurobiology at Yale, and was founder and Director of the Kavli Institute for Neuroscience. He is best known for elucidating the mechanisms involved in development and evolution of the cerebral cortex. In 2008, Rakic shared the inaugural Kavli Prize in Neuroscience. He is currently the Dorys McConell Duberg Professor of Neuroscience, leads an active research laboratory, and serves on Advisory Boards and Scientific Councils of a number of Institutions and Research Foundations.
The development of the nervous system in humans, or neural development or neurodevelopment involves the studies of embryology, developmental biology, and neuroscience to describe the cellular and molecular mechanisms by which the complex nervous system forms in humans, develops during prenatal development, and continues to develop postnatally.

The ganglionic eminence (GE) is a transitory structure in the development of the nervous system that guides cell and axon migration. It is present in the embryonic and fetal stages of neural development found between the thalamus and caudate nucleus.
Gyrification is the process of forming the characteristic folds of the cerebral cortex.

T-box, brain, 1 is a transcription factor protein important in vertebrate embryo development. It is encoded by the TBR1 gene. This gene is also known by several other names: T-Brain 1, TBR-1, TES-56, and MGC141978. TBR1 is a member of the TBR1 subfamily of T-box family transcription factors, which share a common DNA-binding domain. Other members of the TBR1 subfamily include EOMES and TBX21. TBR1 is involved in the differentiation and migration of neurons and is required for normal brain development. TBR1 interacts with various genes and proteins in order to regulate cortical development, specifically within layer VI of the developing six-layered human cortex. Studies show that TBR1 may play a role in major neurological diseases such as Alzheimer's disease (AD), Parkinson's disease (PD) and autism spectrum disorder (ASD).

A cerebral organoid, or brain organoid, describes an artificially grown, in vitro, miniature organ resembling the brain. Cerebral organoids are created by culturing pluripotent stem cells in a three-dimensional rotational bioreactor, and they develop over a course of months. The brain is an extremely complex system of heterogeneous tissues and consists of a diverse array of neurons. This complexity has made studying the brain and understanding how it works a difficult task in neuroscience, especially when it comes to neurodegenerative diseases. The purpose of creating an in vitro neurological model is to study these diseases in a more simple and variable space. This 3D model is free of many potential in vivo limitations. The varying physiology between human and other mammalian models limits the scope of study in neurological disorders. Cerebral organoids are synthesized tissues that contain several types of nerve cells and have anatomical features that recapitulate regions of the cortex observed in brains. Cerebral organoids are most similar to layers of neurons called the cortex and choroid plexus. In some cases, structures similar to the retina, meninges and hippocampus can form. Stem cells have the potential to grow into many different types of tissues, and their fate is dependent on many factors. Below is an image showing some of the chemical factors that can lead stem cells to differentiate into various neural tissues; a more in-depth table of generating specific organoid identity has been published since. Similar techniques are used on stem cells used to grow cerebral organoids.
Cajal–Retzius cells are a heterogeneous population of morphologically and molecularly distinct reelin-producing cell types in the marginal zone/layer I of the developmental cerebral cortex and in the immature hippocampus of different species and at different times during embryogenesis and postnatal life.

Susan McConnell is a neurobiologist who studies the development of neural circuits in the mammalian cerebral cortex. She is a professor in the Department of Biology at Stanford University, where she is the Susan B. Ford Professor of Humanities and Sciences, a Bass University Fellow, and a Howard Hughes Medical Institute Professor. She is an elected member of the National Academy of Sciences and the American Academy of Arts and Sciences.
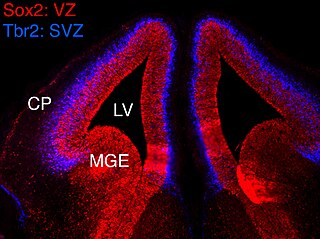
In vertebrates, the ventricular zone (VZ) is a transient embryonic layer of tissue containing neural stem cells, principally radial glial cells, of the central nervous system (CNS). The VZ is so named because it lines the ventricular system, which contains cerebrospinal fluid (CSF). The embryonic ventricular system contains growth factors and other nutrients needed for the proper function of neural stem cells. Neurogenesis, or the generation of neurons, occurs in the VZ during embryonic and fetal development as a function of the Notch pathway, and the newborn neurons must migrate substantial distances to their final destination in the developing brain or spinal cord where they will establish neural circuits. A secondary proliferative zone, the subventricular zone (SVZ), lies adjacent to the VZ. In the embryonic cerebral cortex, the SVZ contains intermediate neuronal progenitors that continue to divide into post-mitotic neurons. Through the process of neurogenesis, the parent neural stem cell pool is depleted and the VZ disappears. The balance between the rates of stem cell proliferation and neurogenesis changes during development, and species from mouse to human show large differences in the number of cell cycles, cell cycle length, and other parameters, which is thought to give rise to the large diversity in brain size and structure.
Cortical patterning is a field of developmental neuroscience which aims to determine how the various functional areas of the cerebral cortex are generated, what size and shape they will be, and how their spatial pattern across the surface of the cortex is specified. Early brain lesion studies indicated that different parts of the cortex served different cognitive functions, such as visual, somatosensory, and motor functions, beautifully assimilated by Brodmann in 1909. Today the field supports the idea of a 'protomap', which is a molecular pre-pattern of the cortical areas during early embryonic stages. The protomap is a feature of the cortical ventricular zone, which contains the primary stem cells of the cortex known as radial glial cells. A system of signaling centers, positioned strategically at the midline and edges of the cortex, produce secreted signaling proteins that establish concentration gradients in the cortical primordium. This provides positional information for each stem cell, and regulates proliferation, neurogenesis, and areal identity. After the initial establishment of areal identity, axons from the developing thalamus arrive at their correct cortical areal destination through the process of axon guidance and begin to form synapses. Many activity-dependent processes are then thought to play important roles in the maturation of each area.

The Radial Unit Hypothesis (RUH) is a conceptual theory of cerebral cortex development, first described by Pasko Rakic. The RUH states that the cerebral cortex develops during embryogenesis as an array of interacting cortical columns, or 'radial units', each of which originates from a transient stem cell layer called the ventricular zone, which contains neural stem cells known as radial glial cells.
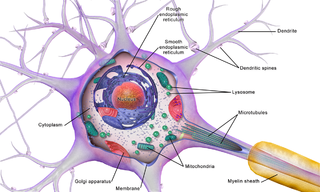
Brain cells make up the functional tissue of the brain. The rest of the brain tissue is structural or connective called the stroma which includes blood vessels. The two main types of cells in the brain are neurons, also known as nerve cells, and glial cells also known as neuroglia.
References
- 1 2 Rakic P (July 1988). "Specification of cerebral cortical areas". Science. 241 (4862): 170–6. doi:10.1126/science.3291116. PMID 3291116.
- ↑ Noctor, SC; Flint, AC; Weissman, TA; Dammerman, RS; Kriegstein, AR (8 February 2001). "Neurons derived from radial glial cells establish radial units in neocortex". Nature. 409 (6821): 714–20. doi:10.1038/35055553. PMID 11217860. S2CID 3041502.
- ↑ Rakic, P (October 2009). "Evolution of the neocortex: a perspective from developmental biology". Nature Reviews. Neuroscience. 10 (10): 724–35. doi:10.1038/nrn2719. PMC 2913577 . PMID 19763105.
- ↑ Grove, EA; Fukuchi-Shimogori, T (2003). "Generating the cerebral cortical area map". Annual Review of Neuroscience. 26: 355–80. doi:10.1146/annurev.neuro.26.041002.131137. PMID 14527269. S2CID 12282525.
- 1 2 Fukuchi-Shimogori, T; Grove, EA (2 November 2001). "Neocortex patterning by the secreted signaling molecule FGF8". Science. 294 (5544): 1071–4. doi: 10.1126/science.1064252 . PMID 11567107. S2CID 14807054.
- ↑ Sur, M; Rubenstein, JL (4 November 2005). "Patterning and plasticity of the cerebral cortex". Science. 310 (5749): 805–10. doi:10.1126/science.1112070. PMID 16272112. S2CID 17225116.
- ↑ O'Leary, DD (October 1989). "Do cortical areas emerge from a protocortex?". Trends in Neurosciences. 12 (10): 400–6. doi:10.1016/0166-2236(89)90080-5. PMID 2479138. S2CID 9371858.
- ↑ Cognitive Neuroscience of Development (Studies in Developmental Psychology). East Sussex: Psychology Press. 2003. ISBN 978-1-84169-214-2.
- ↑ Sun T, Walsh CA (August 2006). "Molecular approaches to brain asymmetry and handedness". Nature Reviews Neuroscience. 7 (8): 655–62. doi:10.1038/nrn1930. PMID 16858393. S2CID 12208186.
Glossary
{{cite journal}}: External link in|quote= - ↑ Bishop, KM; Goudreau, G; O'Leary, DD (14 April 2000). "Regulation of area identity in the mammalian neocortex by Emx2 and Pax6". Science. 288 (5464): 344–9. doi:10.1126/science.288.5464.344. PMID 10764649.
- ↑ Mallamaci, A; Muzio, L; Chan, CH; Parnavelas, J; Boncinelli, E (July 2000). "Area identity shifts in the early cerebral cortex of Emx2-/- mutant mice". Nature Neuroscience. 3 (7): 679–86. doi:10.1038/76630. PMID 10862700. S2CID 20047258.
- ↑ Ackman, JB; Burbridge, TJ; Crair, MC (11 October 2012). "Retinal waves coordinate patterned activity throughout the developing visual system". Nature. 490 (7419): 219–25. doi:10.1038/nature11529. PMC 3962269 . PMID 23060192.